England Becomes First in Europe to Offer Cancer Patients a Fast-Track Immunotherapy Injection
England Becomes First in Europe to Offer Cancer Patients a Fast-Track Immunotherapy Injection
Up to 15,000 Cancer Patients Annually to Benefit from Nivolumab Jab for 15 Types of Cancer
Cancer patients in England are now the first in Europe to benefit from a new, faster method of immunotherapy treatment. NHS England has announced that up to 15,000 patients each year could receive nivolumab (Opdivo) in injectable form, offering a quicker and more convenient alternative to traditional intravenous (IV) infusions.
What Is Nivolumab and How Does It Work?
Nivolumab is a groundbreaking immunotherapy drug that helps the immune system target and destroy cancer cells. It works by blocking the PD-1 protein on T-cells, allowing the body’s immune system to recognize and attack tumor cells more effectively.
This treatment is suitable for 15 different types of cancer, including:
-
Lung cancer
-
Bowel (colorectal) cancer
-
Kidney cancer
-
Bladder cancer
-
Esophageal cancer
-
Skin cancer (including melanoma)
-
Head and neck cancers
Faster, Easier Treatment for Cancer Patients
The injectable version of nivolumab takes only 3 to 5 minutes to administer, compared to the up to one hour needed for IV infusions. With most patients receiving treatment every two to four weeks, this new method is expected to save over a year of treatment time annually across the NHS.
According to Professor Peter Johnson, NHS England’s national clinical director for cancer, the shift to injectable immunotherapy will not only improve patient convenience but also free up hospital capacity and allow more patients to be treated.
Approved and Cost-Effective
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has approved the injectable form of nivolumab. NHS England confirmed that the cost of the jab will remain the same as the IV version, following a price agreement with manufacturer Bristol Myers Squibb.
A Step Forward in Cancer Innovation
Naser Turabi, Director of Evidence and Implementation at Cancer Research UK, emphasized the importance of innovations like injectable immunotherapy in delivering faster, more efficient care. “We’re in a golden age of cancer research,” he said. “It’s vital our healthcare system evolves to keep up with these advancements.”
The NHS plans to offer this injectable form to around 1,200 patients per month, with most new patients likely to receive this updated treatment method.
A Bright Future for Cancer Care in England
This major step marks another milestone in making cutting-edge cancer treatments more accessible across the UK. With continued investment, the upcoming national cancer plan for England is expected to further strengthen cancer care services, enabling quicker access to lifesaving therapies.
News in the same category


Sleeping on your left side affects your health in ways you would have never thought
After Being Diagnosed With Dementia at 49, Man Realized The Subtle Red Flag in His Work That Made Him Realize Something Was Wrong

🌿 18 Reasons Why Oregano (Orégano Orejón) Should Be a Staple in Your Home

🌬️ Persistent Cough, Mucus Buildup, or Lung Congestion? Try This Powerful Natural Onion Remedy
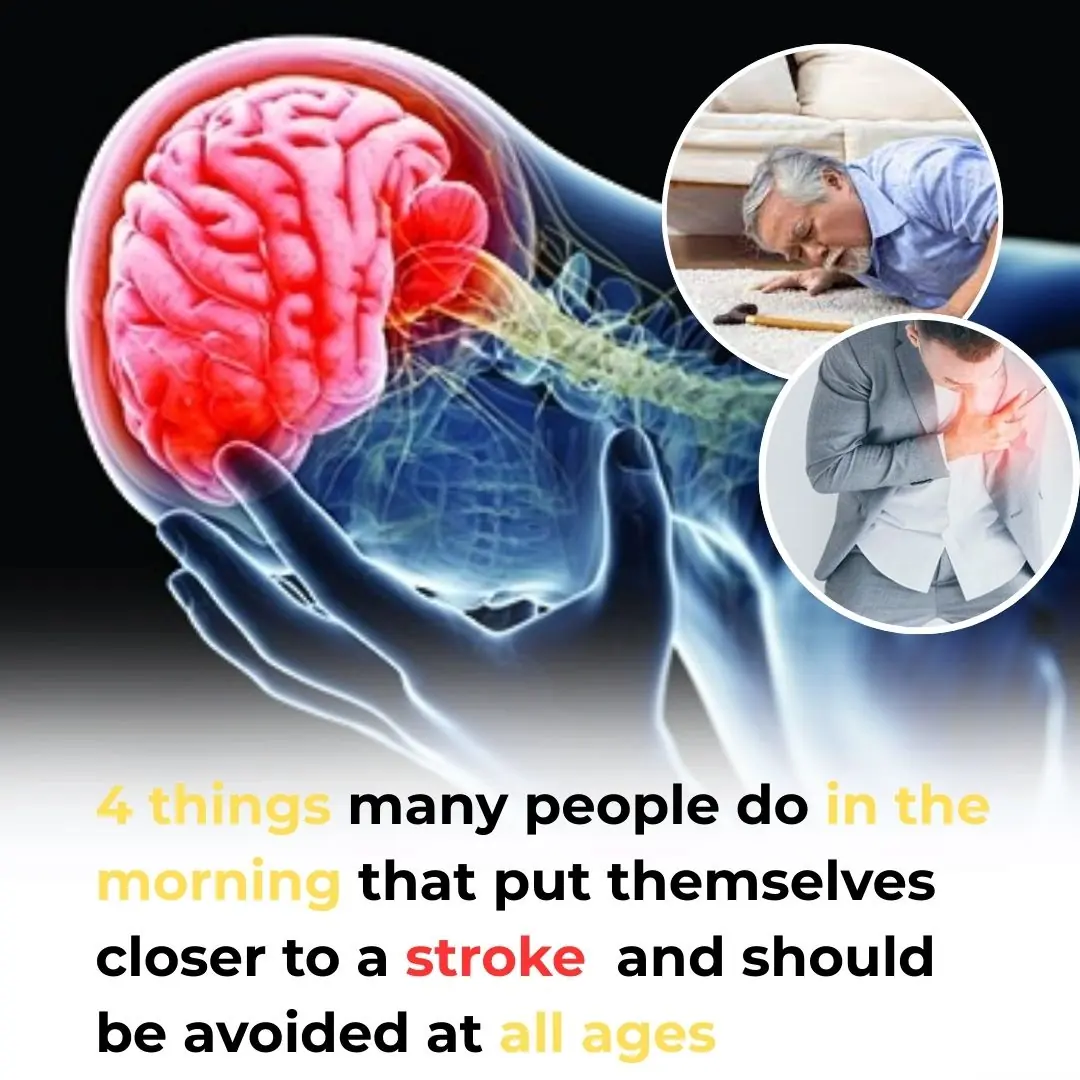
4 common morning habits that may increase your risk of stroke

This Herbal Tea Can Help with Diabetes, Liver Health, High Blood Pressure, and Poor Circulation

🍹 Boost Your Body Naturally: 6 Juice Recipes for Common Health Issues

Vitamin K Precursor Found to Target and Destroy Cancer Cells in Latest Research
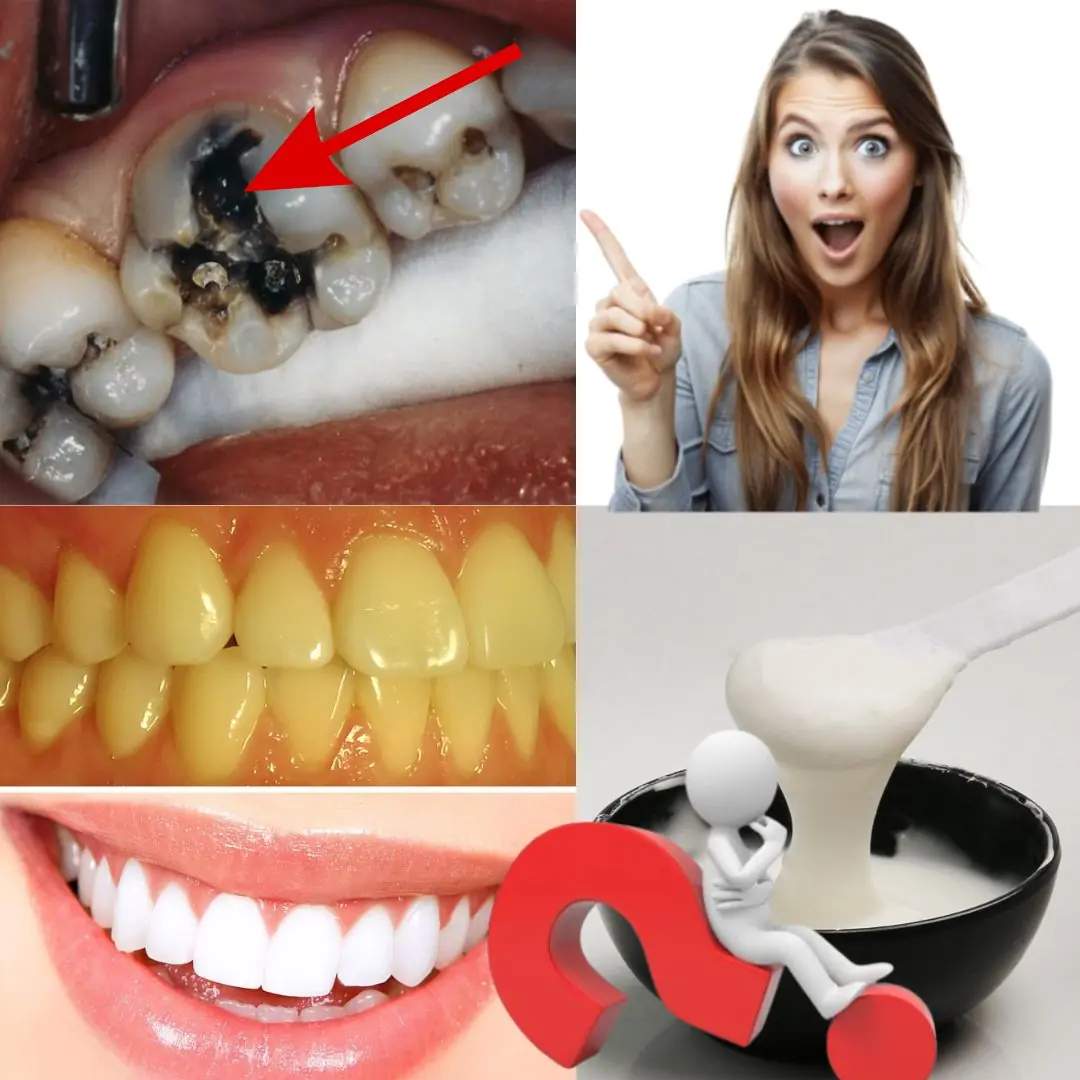
Naturally Reverse Early Tooth Decay: 6 Proven Tips to Strengthen Enamel and Fight Cavities!

The Beauty Benefits of a Coffee and Vaseline Face Mask: A Natural Wrinkle-Reducer?
30 Incredible Benefits of Dandelion: Nature’s Hidden Gem

Sage: A Natural Remedy for Brain Health, Inflammation, and Joint Pain

Child Actor Passes Away After Repeated Heart Attacks As Family Claim Doctors Failed To Diagnose Symptoms Correctly
Pregnancy Changes the Brain: How Motherhood Transforms the Mind

4 types of people who should avoid eating cabbage

The #1 Anti-Cancer Food You're Not Eating (But Should!)

Discover the Untapped Potential of Chili Pepper Leaves: Nutritional Powerhouse for Your Health and Kitchen

Plantar Warts and Skin Tags Disappear Overnight with this recipe
News Post

My MIL Mocked Me for Making My Own Wedding Cake – Then Took Credit for It in Her Speech

At My Sister's Wedding, My Son Grabbed My Hand and Whispered, 'Mom… We Need to Go. Now!' – What He Showed Me Changed Everything

I Visited My Sister, Was Shocked to See Who Her Fiancé Was, and Knew I Couldn't Let It Go That Easily

My New Neighbor Was the Perfect Man Next Door Until I Overheard His Plan Against Me

My Daughter-in-Law Threw Out Most of My Kitchen Utensils—So I Brought Her Back Down to Earth

5 Early Cancer Symptoms You Must Not Overlook

Sleeping on your left side affects your health in ways you would have never thought

After Being Diagnosed With Dementia at 49, Man Realized The Subtle Red Flag in His Work That Made Him Realize Something Was Wrong

Astronomer Rides Simulation To The Edge Of The Universe—Chasing Light From The Big Bang

🌿 18 Reasons Why Oregano (Orégano Orejón) Should Be a Staple in Your Home

Controversial Inventor’s Mysterious Death Sparks Debate Over Alternative Energy Suppression

🌬️ Persistent Cough, Mucus Buildup, or Lung Congestion? Try This Powerful Natural Onion Remedy

4 common morning habits that may increase your risk of stroke

This Herbal Tea Can Help with Diabetes, Liver Health, High Blood Pressure, and Poor Circulation

🍹 Boost Your Body Naturally: 6 Juice Recipes for Common Health Issues

Man Dips Finger In Yellowstone Hot Spring, Accidentally Falls In And Dissolves Within A Day

Vitamin K Precursor Found to Target and Destroy Cancer Cells in Latest Research

Naturally Reverse Early Tooth Decay: 6 Proven Tips to Strengthen Enamel and Fight Cavities!

The Beauty Benefits of a Coffee and Vaseline Face Mask: A Natural Wrinkle-Reducer?
