
Game-Changer: England Officially Rolls Out New Injections That Fights 15 Types of Cancer
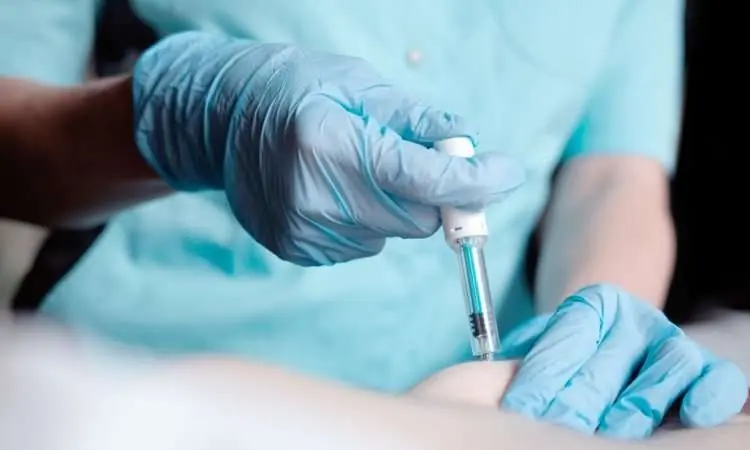
In a pivotal moment for cancer care, England has officially introduced a fast-track injectable treatment for cancer patients, marking a first for any healthcare system in Europe. This innovation takes the form of a newly approved subcutaneous version of the widely used immunotherapy drug, nivolumab - a development that promises to radically improve both efficiency and patient experience.
This new injection isn’t just an update in administration. It represents a leap forward in how cancer care is delivered - replacing the time-consuming, traditional hour-long intravenous infusion with a simple jab in the arm that takes less than five minutes.
And it’s already being hailed as a major milestone.
A Shift That Could Benefit Thousands
The streamlined injectable version of nivolumab is expected to benefit up to 15,000 patients annually across England. With its capability to treat 15 different types of cancer, including lung, bowel, skin, and oesophageal cancers, this development could reshape how immunotherapy is administered nationwide.
What once required patients to spend up to an hour in treatment chairs now only takes three to five minutes - freeing up valuable hospital resources while improving the day-to-day lives of those undergoing cancer therapy.
The Rise of the Five-Minute Immunotherapy
Previously, nivolumab was administered through an intravenous drip - a process that not only took time but also required extensive hospital infrastructure. But with the new subcutaneous injection, patients can receive the same life-saving drug in just minutes.
This change, made possible through regulatory approval and a new agreement between the National Health Service (NHS) and drug manufacturer Bristol Myers Squibb, is expected to save the healthcare system over a year’s worth of collective treatment time annually.
The implications go far beyond saving time. For patients, the reduced treatment burden translates into more time spent at home or work and fewer interruptions to their daily lives. For overburdened hospitals, it means increased capacity to treat more patients without adding staff or equipment.
Understanding the Science Behind Nivolumab
So, how does nivolumab work?
Nivolumab is what scientists call an immune checkpoint inhibitor. It targets the PD-1 protein - a regulatory switch found on T-cells that acts as a brake to prevent the immune system from attacking healthy cells. Unfortunately, cancer cells exploit this system to hide from the immune response.
What nivolumab does is block PD-1, effectively removing the disguise that cancer cells wear to evade immune attack. This enables the immune system to detect and attack the tumor, turning the body’s natural defenses into a potent weapon against cancer.
Produced under the brand name Opdivo by Bristol Myers Squibb, nivolumab has long been a cornerstone in modern oncology. This latest development in delivery marks a new chapter in its clinical application.
Regulatory Approval and Immediate Rollout
The Medicines and Healthcare products Regulatory Agency (MHRA) has approved this injectable formulation, endorsing it as both effective and safe. In response, NHS England has begun rolling out the injection immediately, incorporating it into current cancer care pathways.
According to the NHS, approximately 1,200 patients per month are now eligible to receive the injectable treatment. This figure is expected to rise as more patients are prescribed nivolumab as part of their ongoing treatment.
Professor Peter Johnson, NHS England’s National Clinical Director for Cancer, shared his optimism about the rollout. “Immunotherapy has already been a huge step forward for many NHS patients with cancer,” he said. “And being able to offer it as an injection in minutes means we can make the process far more convenient. It also frees up capacity so that teams can treat even more patients.”
A Cost-Neutral Revolution
One might assume such a transformative treatment would come with a steep price tag. However, NHS England has confirmed that the injection will cost no more than the traditional IV version, thanks to a pricing agreement with Bristol Myers Squibb.
This cost-neutral strategy is a vital component of the rollout. As healthcare systems around the world struggle to balance quality care with tight budgets, maintaining affordability while introducing innovation is an achievement in itself.
A Broad Spectrum of Impact
The injectable version of nivolumab isn’t limited to a few rare cancers. It has been approved for use in a wide array of conditions, including:
- Non-small cell lung cancer
- Melanoma
- Kidney cancer
- Bladder cancer
- Head and neck squamous cell carcinoma
- Oesophageal cancer
- Certain cases of colorectal cancer
This broad application means thousands of patients across different cancer types stand to benefit, making it more than just a specialized treatment - it has the potential to become a new standard in cancer care.
Streamlined Care, Elevated Comfort
Beyond convenience, this innovation also brings significant improvements in patient comfort.
Many cancer patients experience fatigue, anxiety, or physical discomfort. The idea of trading lengthy infusion sessions for a quick injection can provide a psychological boost that shouldn't be underestimated.
Fewer hospital visits also mean fewer days missed at work, less time spent in traffic, and more opportunities to focus on recovery and family life. This human side of treatment efficiency is crucial, yet often under-discussed.
The Bigger Picture: Innovation in Oncology
The rollout of injectable nivolumab is part of a broader shift in the cancer treatment landscape - one that emphasizes personalization, innovation, and quality of life.
Naser Turabi, Director of Evidence and Implementation at Cancer Research UK, highlighted the importance of evolving with science. “Innovations like this will be vital for treating cancer patients sooner and more efficiently,” he said. “We’re in a golden age of cancer research, and it’s essential that our health service continues to adapt to deliver the best possible care for patients.”
Turabi also stressed the need for policy support and long-term planning, urging the government to include sustainable innovation strategies in the upcoming national cancer plan.
The Future of Cancer Care in England
This shift to injectable immunotherapy is more than a procedural update - it symbolizes a larger vision for the future of cancer treatment. It highlights what can happen when medical science, healthcare policy, and pharmaceutical innovation align with the patient’s best interest.
With cancer rates rising and healthcare resources increasingly stretched, streamlined, effective, and patient-centered treatments like this are not just welcome - they’re necessary.
As more countries look to emulate England’s model, this five-minute injection may become a global template for how cancer treatment can evolve - quicker, more comfortable, and accessible to more people than ever before.
News in the same category

Groundbreaking Research: Reversing Memory Loss In Alzheimer’s Disease Without Removing Plaques

Rfk Jr. Raises Health Concerns Over 5G, Says It May Affect Brain Function And Cancer Risk

Eat Just 3 of These Daily and Watch What Happens to Your Body
. Their ability to benefit nearly every major system in the body - from the heart and liver to the brain and bones - makes them a powerful ally in maintaining health and vitality.

Blood Type O Diet: What to Eat and What to Avoid

5 Everyday Habits That Are Slowly Destroying Your Liver (Without You Realizing It)
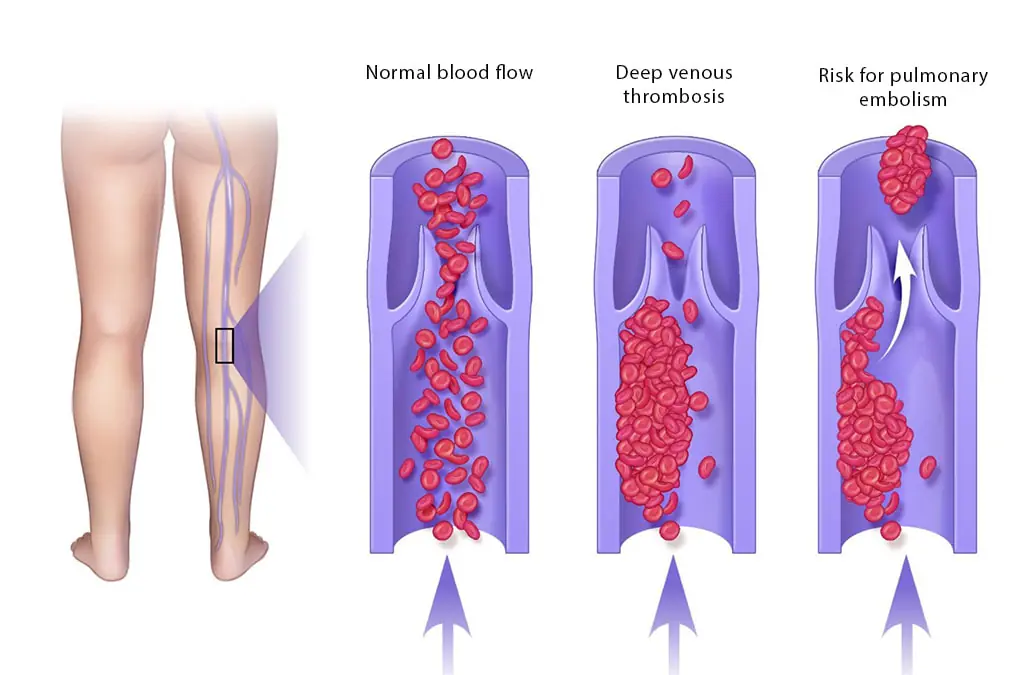
Blood Clot in Leg: Signs and Symptoms You Shouldn’t Ignore
If Your Nails Show These 6 Signs, See a Doctor Immediately
While some alterations may be harmless, others could be early warnings of serious health conditions.
‘Healthy’ 38-year-old shares the only bowel cancer symptom he noticed — And it wasn’t blood in the loo

Young Dad Misses Key Cancer Symptom That Left Him Terrified

5 Things Doctors Say You Should Never Give Your Kids to Help Prevent Cancer
Indiana Boy, 8, Dies Hours After Contracting Rare Brain Infection At School
World-First Gene Therapy Restores Sight to Boy Born Blind

A Warning About The ‘Worst Thing’ That People Should Never Do When Awakening in the Night

Horrifying simulation shows life-threatening impact of drinking too much water and how it can lead to death
These simulations, tragic stories, and medical data remind us that moderation matters.
Cancer Breakthrough: Scientists Discover Protein That Puts Tumors to Sleep!
With promising results in preclinical trials and a growing understanding of the tumor microenvironment, type III collagen may become a key player in the fight against cancer - not by eradicating it, but by keeping it permanently asleep.

The Amazing Health Benefits and Uses of Castor Oil

Evidence-Based Health Benefits of Raw, Pure, Natural Honey + Turmeric Golden Honey Recipe
Home Remedies for Blocked and Stuffy Nose
News Post

She held on, certain they would come back. Yet as days turned into weeks, her strength waned—until a fresh kind of love unexpectedly entered her life.

The Hidden Light In Your Hands That Shouldn’t Be Dimmed

Mosquitoes Rain From The Sky Over Hawaii—Scientists Explain Why

Doctor Shares 30-Second Hand Test That Could Reveal Hidden Brain Tumor

Nasa Tracks Plane-Sized Asteroid Speeding Toward Earth At 47,000 Mph

Groundbreaking Research: Reversing Memory Loss In Alzheimer’s Disease Without Removing Plaques

Rfk Jr. Raises Health Concerns Over 5G, Says It May Affect Brain Function And Cancer Risk

Two-Year-Old Boy Bites Cobra To Death After Snake Coils Around His Hands In India

Four Famous Psychics Warn World War III Could Begin By End Of 2025

Abandoned puppy was found in terrible shape — see his incredible transformation one year later in the comments 🥹

Eat Just 3 of These Daily and Watch What Happens to Your Body
. Their ability to benefit nearly every major system in the body - from the heart and liver to the brain and bones - makes them a powerful ally in maintaining health and vitality.

Psychic who suffered near-death experience reveals shocking truth about the afterlife
A psychic has detailed about afterlife after claiming to have experienced the feeling "dea@th" four times.

Blood Type O Diet: What to Eat and What to Avoid

5 Everyday Habits That Are Slowly Destroying Your Liver (Without You Realizing It)

Blood Clot in Leg: Signs and Symptoms You Shouldn’t Ignore

Real story behind Amelia Earhart and the Bermuda Triangle as scientists 'solve mystery' after 88 years

Airlines forced to cancel flights after 'Japanese Baba Vanga' predicts catastrophic disaster

8.7 magnitude earthquake off Russia: Japan experiences first tsunami, many countries issue warnings

After Seeing Her Husband's Photo with a Pregnant Stranger, Nadya Chose Silence – But What Happened Next Was Sh0cking
It was unthinkable! How could this be happening? Nadezhda thought, staring at a photograph of a young pregnant woman sitting on her husband’s lap, smiling, and clinging to him. Twenty-five years of marriage... and now this? Her heart tightened painfully

I Went Undercover as a Homeless Person to Test My Granddaughter's Fiancé – What I Found Was Beyond Sh0cking
I dressed in old, ragged clothes, hid my face beneath a weathered hat, and stood on the street like a beggar—just to see what kind of man my granddaughter was marrying. I thought I was prepared for anything, but what happened next left me speechless and