
Injectable Gel Breakthrough Brings New Hope for Nerve Regeneration
Researchers are reporting a remarkable breakthrough that could reshape the future of nerve-injury treatment. According to early laboratory studies, a team associated with MIT has developed an injectable regenerative gel capable of stimulating damaged nerves to regrow and potentially restoring lost sensation. While the findings come primarily from controlled animal experiments, they highlight a rapidly advancing field of biomedical engineering that is pushing the boundaries of what nerve repair might look like in the years ahead.
The technology centers on a specially engineered hydrogel—a soft, biocompatible material that can be delivered directly to the site of nerve injury through a minimally invasive injection. Once inside the body, the gel forms a supportive three-dimensional scaffold that mimics natural extracellular tissue. This environment allows severed or damaged nerve fibers to reconnect and extend, while embedded biochemical molecules help guide their direction of growth. Studies in journals such as Advanced Materials and Nature Biomedical Engineering have shown that hydrogels with tailored mechanical and chemical properties can significantly accelerate axonal regrowth and reduce harmful scar formation, which is one of the biggest obstacles in nerve repair.
Early reports suggest that animals treated with the gel regained substantial sensation within just a few weeks—an outcome rarely seen with standard recovery treatments. In traditional nerve injuries, regrowth is often slow, incomplete, or blocked entirely by scar tissue. However, this gel appears to promote both structural regeneration and functional restoration. Similar results have been observed in other preclinical research, such as spinal cord repair studies conducted at Rowan University and discussed in ScienceDaily, where biomaterial scaffolds helped reconnect previously damaged neural pathways.
If this approach proves effective in humans, the implications would be profound. Instead of relying solely on complex surgical grafts, nerve transfers, or implanted electrical stimulators, doctors could one day administer an injection that triggers the body’s own repair processes. This could transform treatment for a wide range of conditions, including peripheral nerve trauma, spinal cord injuries, diabetic neuropathy, and even certain types of sensory loss.
Scientists emphasize, however, that this work is still in the early stages. Human clinical trials have not yet begun, and many questions remain about long-term safety, stability of regenerated nerves, and how well results from animal models will translate to people. Publications in Frontiers in Bioengineering and Biotechnology and Journal of Neural Engineering also stress that regenerative gels must be carefully tuned to avoid inflammation, unwanted tissue growth, or incomplete integration with existing nerve structures.
Despite these uncertainties, the momentum behind regenerative biomaterials is stronger than ever. Research teams worldwide are investigating injectable hydrogels, stem-cell–infused scaffolds, and bioactive polymers as next-generation therapies for nerve repair. With continued development, technologies like the MIT-associated gel may bring us closer to a future where repairing nerves—and even reversing paralysis—is not only possible but routinely achievable.
Sources (reputable and related to nerve-repair biomaterials):
-
Nature Biomedical Engineering: Biomaterial scaffolds for nerve regeneration
-
Advanced Materials: Injectable hydrogels for neural repair
-
Frontiers in Bioengineering and Biotechnology: Regenerative biomaterials and nerve healing mechanisms
-
Rowan University regenerative biomaterial research (2025), ScienceDaily
-
Journal of Neural Engineering: Advances in peripheral and central nerve repair technologies
News in the same category

Breakthrough Research Suggests Kidney Damage May Be Reversible After All
How Intermittent Fasting Protects the Heart: New Evidence on Blood Clots and Cardiovascular Health

New Evidence Links Hepatitis C to Brain Pathways in Mental Illness

If You See a Woman Wearing a Wedding Ring On Her Pinky, Here's What It Means

Reinventing Renewable Energy: Germany Launches Compact Turbine for Off-Grid Power

Rethinking Depression: New Brain-Imaging Research Reveals It’s More Than a Chemical Imbalance

Seventeen Years Lost: How a Look-Alike Helped Free an Innocent Man

You Must Live Without One Modern Comfort — Your Choice Reveals Who You Really Are

Why do foreigners use electric kettles so little even though they are very convenient?

How Poor Sitting Posture Impacts Your Spine, Muscles, and Overall Health
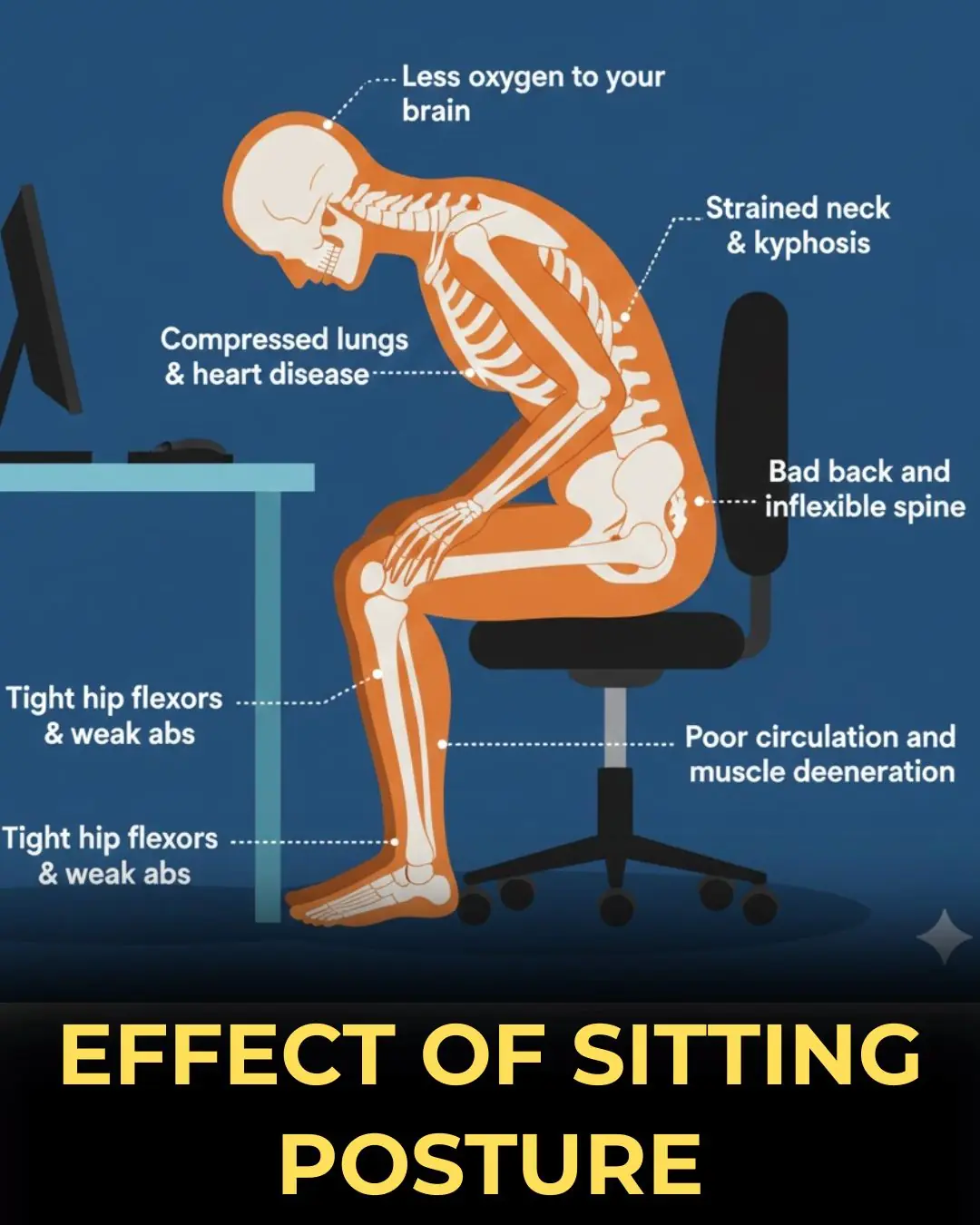
Understanding the Long-Term Consequences of Poor Sitting Posture
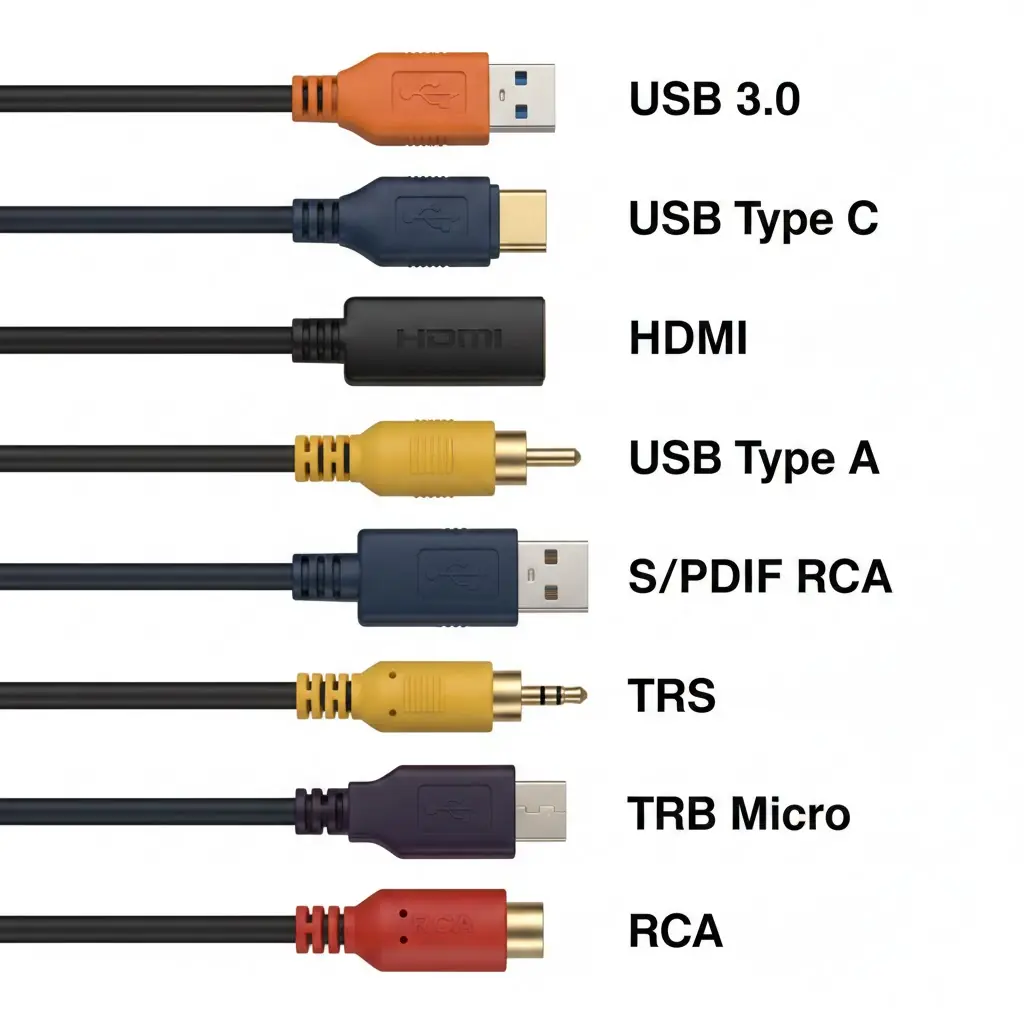
A Complete Guide to Common Cable Types and How They Keep Devices Connected

Egg Freshness Explained: What Sinking, Tilting, and Floating Really Mean

A Sleeping Giant Stirs: Taftan Volcano Experiences Uplift Driven by Shallow Gas Pressure

The Healing Power of Touch: How Hugs Support Emotional Balance and Immune Health

Why Sleeping in Socks Might Be the Secret to Better Sleep

Think Bottled Water Is Safer Think Again

Can Eggs Protect Your Mind? Emerging Evidence Suggests a Cognitive Benefit
News Post

Why Boiled Eggs Deserve a Spot on Your Breakfast Table

Goodbye Synthetic Dyes: Doritos Join the Push for Cleaner, Transparent Ingredients

Breakthrough Research Suggests Kidney Damage May Be Reversible After All

How Intermittent Fasting Protects the Heart: New Evidence on Blood Clots and Cardiovascular Health

New Evidence Links Hepatitis C to Brain Pathways in Mental Illness

ITV breaks silence as Celebrity Big Brother is ‘axed from ITV schedule’

Peter Andre teases ‘special’ project with wife Emily: ‘We are having exciting meetings’

🚫 When to Avoid Ginger — 6 Medical Conditions That May Be Affected

What Happens to Your Body When You Eat Canned Tuna Every Day

I’m A Celebrity star Kelly Brook’s husband reveals when he’s flying out to Australia

Kris Jenner shows support for Meghan Markle weeks after Kardashians photo scandal

Inside Angry Ginge’s ‘bromance’ with Angry Ginge – how they met; ‘going to war’ over diss track; huge ‘risk’ that ‘paid off’

Ant McPartlin’s tattoos explained – tribute to wife Anne-Marie; uproar over ‘missing’ family member; nod to his recovery

Emmerdale disaster incoming: Bear’s fate ‘sealed’ as Joshua Richards makes devastating admission

How Do Farmers Grow Avocado Trees

Robron plot Kev’s downfall – but Emmerdale fans declare they ‘love him’

If You See a Woman Wearing a Wedding Ring On Her Pinky, Here's What It Means

2-Minute Painless Hair Removal: Natural At-Home Solution
