
Promising Early Results for ELI-002 2P: A New Vaccine Targeting Pancreatic Cancer
Promising Results for New Pancreatic Cancer Vaccine in Early Clinical Trial
A groundbreaking new cancer vaccine, named ELI-002 2P, has demonstrated promising outcomes in an early-phase clinical trial for patients diagnosed with pancreatic cancer. The vaccine is specifically designed to stimulate the immune system, training it to target and eliminate cancer cells, particularly those harboring mutations in the KRAS gene. This genetic mutation is prevalent in up to 90% of pancreatic cancer cases, making it a key target for therapeutic intervention.
In the clinical trial, patients who were administered the vaccine following surgery showed robust immune responses. Some patients even exhibited a substantial reduction in the risk of cancer recurrence. This suggests that ELI-002 2P could potentially enhance long-term survival by preventing the return of the disease after surgical intervention. The vaccine’s mechanism is rooted in its ability to "teach" the immune system to recognize and destroy cancer cells expressing mutated KRAS proteins, which are common in pancreatic tumors.
One of the most notable features of ELI-002 2P is its off-the-shelf nature. Unlike personalized cancer therapies that require custom-tailored treatments based on a patient's specific genetic makeup, this vaccine can be produced in advance and administered to patients without modifications. This makes the vaccine potentially more accessible and cost-effective compared to other immunotherapies, which could significantly reduce the financial burden on both patients and healthcare systems.
However, while the initial results are encouraging, experts emphasize the importance of conducting larger, more comprehensive studies to further evaluate the vaccine's efficacy and safety profile. Early-phase trials often focus on determining the treatment's safety, and while promising, the long-term effects and broader applicability of the vaccine remain to be confirmed in larger cohorts of patients.
Researchers are optimistic about the potential of ELI-002 2P as an important tool in the battle against pancreatic cancer, a notoriously aggressive and difficult-to-treat cancer. Pancreatic cancer remains one of the deadliest forms of cancer, with limited treatment options and poor survival rates. The development of this vaccine brings renewed hope to patients, offering the possibility of a therapeutic breakthrough that could significantly improve outcomes for those affected by this challenging disease.
According to a study published in The New England Journal of Medicine (PMID: 40790272), immune therapies targeting KRAS mutations are at the forefront of cancer research, with similar approaches being explored for other cancers. Such advances may not only provide more effective treatments for pancreatic cancer but also pave the way for broader applications in oncology.
As research continues, experts urge caution and call for more data to confirm the vaccine's effectiveness in larger, more diverse populations. The hope is that as clinical trials progress, ELI-002 2P could become a crucial part of the therapeutic landscape for pancreatic cancer, offering patients new hope for better survival rates and improved quality of life.
Sources:
-
The New England Journal of Medicine – A study on the role of KRAS mutation-targeted immunotherapies. PMID: 40790272
-
Cancer Research UK – Ongoing advancements in pancreatic cancer treatment.
-
American Cancer Society – Insights on pancreatic cancer survival rates and emerging therapies.
News in the same category


Study Finds a Mother’s Early Bond With Her Baby Can Shape a Child’s Sleep for Years

Gramma the Tortoise: A Remarkable Life Spanning Three Centuries

California Surpasses Japan to Become the Fourth-Largest Economy in the World

Why Aging Skin Develops a Distinct Body Odor—and What Science Says Can Help

Photographer Captures Dream Shot of Full Moon Over Christ the Redeemer After Three Years of Effort

China Successfully Tests 3D-Printed Micro Turbojet Engine in Landmark Flight Achievement

Swiss Researchers Trial Blood Filtration Device for Alzheimer's Treatment

Bear Takes Over Truckee Diner in Hilarious Culinary Heist

Many people believe they need to walk ten thousand steps daily to stay healthy

The Quieting Skies: A Stark Decline in North America's Bird Population

Sea Otter Takes Over Santa Cruz Surf Scene: A Bold and Unpredictable Presence
Australia's Revolutionary Bionic Eye: A New Era in Restoring Vision

What does it symbolize when a person who passed away appears in your dream

Tiny Pumpkin Toadlet Discovered in Brazil's Atlantic Forest: A New Species of Vibrantly Colored Frog

Overview Energy's Bold Plan to Beam Power from Space to Earth Using Infrared Lasers

Japan’s Ghost Homes Crisis: 9 Million Vacant Houses Amid a Shrinking Population

Japan’s Traditional Tree-Saving Method: The Beautiful and Thoughtful Practice of Nemawashi

Swedish Billionaire Buys Logging Company to Save Amazon Rainforest
The Farmer Who Cut Off His Own Finger After a Snake Bite: A Tale of Panic and Misinformation
News Post

25 Remarkable Benefits of Guava Leaves and How to Use Them Safely

Study Finds This Popular Sweetener Damages the Brain’s Protective Barrier

Study Finds a Mother’s Early Bond With Her Baby Can Shape a Child’s Sleep for Years

Gramma the Tortoise: A Remarkable Life Spanning Three Centuries

California Surpasses Japan to Become the Fourth-Largest Economy in the World

Why Aging Skin Develops a Distinct Body Odor—and What Science Says Can Help

Photographer Captures Dream Shot of Full Moon Over Christ the Redeemer After Three Years of Effort

China Successfully Tests 3D-Printed Micro Turbojet Engine in Landmark Flight Achievement

Swiss Researchers Trial Blood Filtration Device for Alzheimer's Treatment

Bear Takes Over Truckee Diner in Hilarious Culinary Heist

Many people believe they need to walk ten thousand steps daily to stay healthy

The Quieting Skies: A Stark Decline in North America's Bird Population
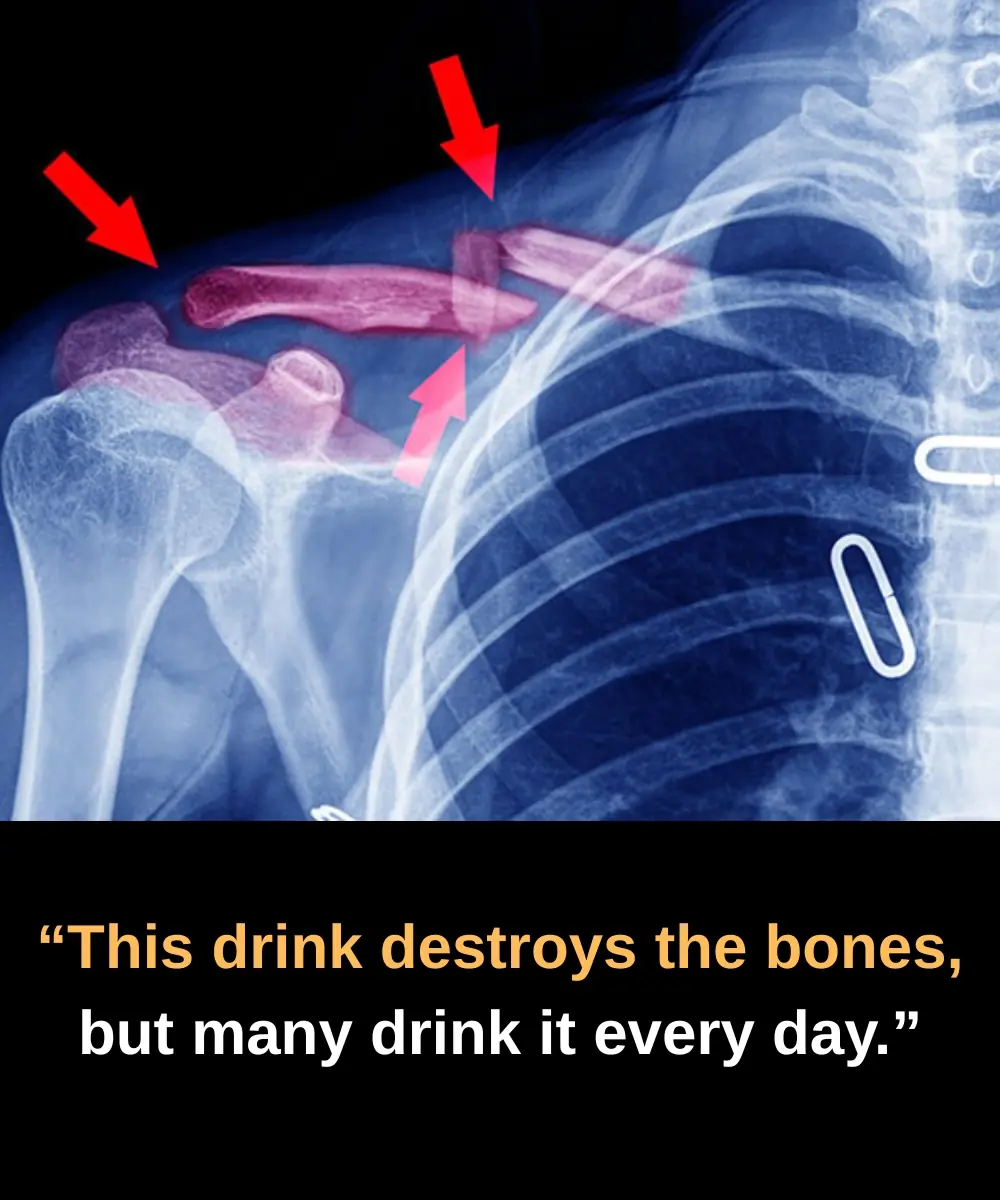
Does This Drink Really Harm Your Bones? The Truth Behind Soda and Your Health

Oregano: The Small Plant with Big Health Benefits

Sea Otter Takes Over Santa Cruz Surf Scene: A Bold and Unpredictable Presence

Australia's Revolutionary Bionic Eye: A New Era in Restoring Vision

10 Best Collagen Gels For Wrinkle Free Glowing Skin

Why You Might Want to Stop Removing Tomato Suckers: What Gardeners Are Starting to Realize

Banana Blossom: Health Benefits, Recipes, and Traditional Uses
