
Is the Green Ring on Your Egg Dangerous? Experts Reveal the Truth
If you are one of those people who prefer their eggs hard-boiled, you have certainly noticed that green color ring around the yolk.
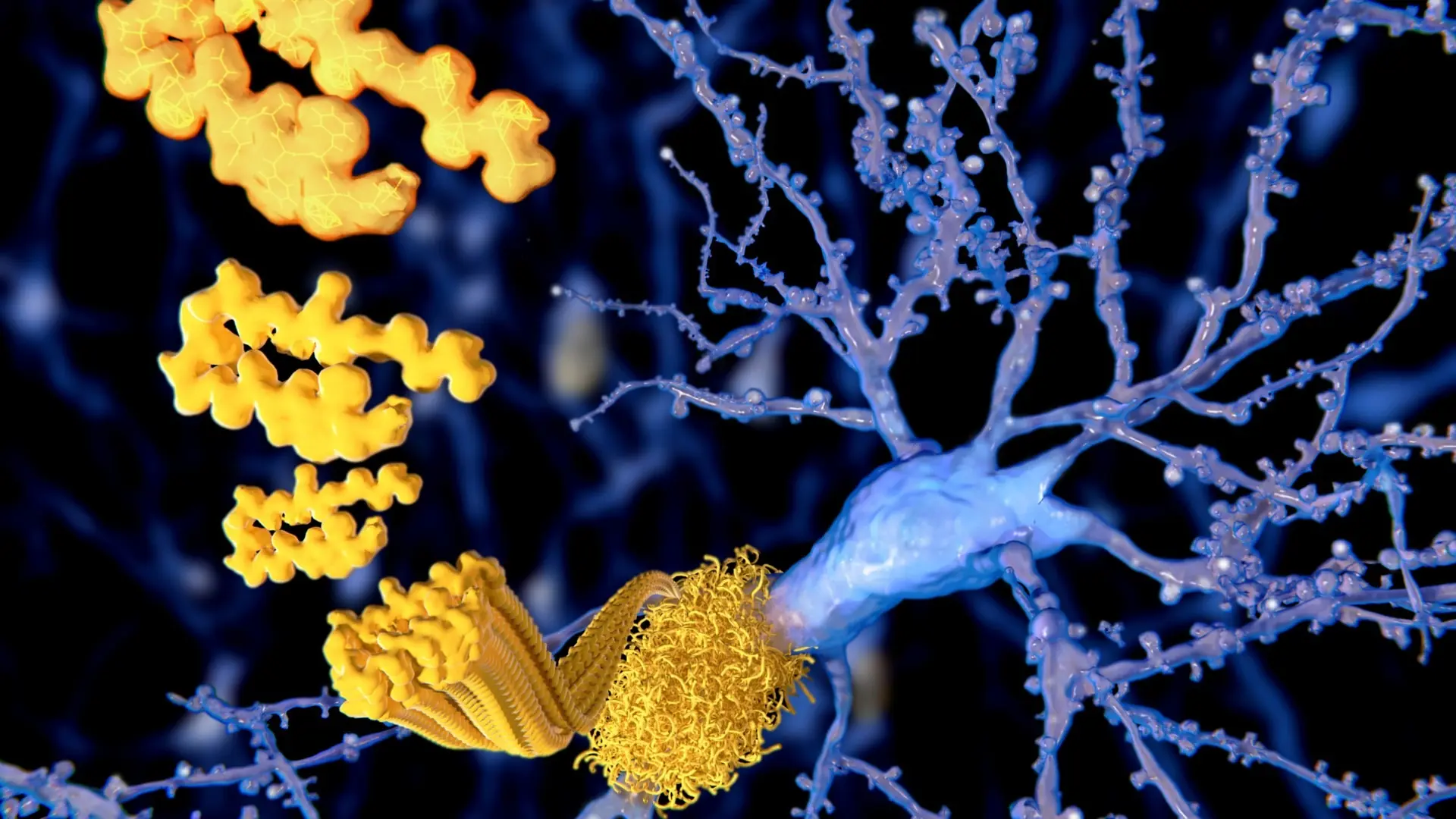
In a groundbreaking shift in how scientists view Alzheimer’s disease, a new model proposes that the condition may stem from just one underlying cause: stress granules. These are tiny, temporary clumps made up of proteins and RNA that form within cells when they are exposed to stress—whether due to environmental triggers or genetic factors.
A team of researchers at Arizona State University believes these microscopic structures may play a much more central role than previously thought. Their theory is based on extensive analysis of medical data and existing literature, including a pivotal 2022 study that tracked the progression of Alzheimer’s. Their findings revealed widespread and consistent changes in gene activity that align with the presence and behavior of stress granules.

When Stress Granules Stay Too Long
Normally, stress granules are part of a healthy cellular response—they act as a defense mechanism to help cells pause nonessential activities during stress and recover afterward. However, new evidence suggests that in the brains of individuals with Alzheimer’s, these granules don’t dissolve as they should. Instead, they linger far beyond the stress event, and that abnormal persistence appears to interfere with critical cellular functions.
One of the most crucial disruptions involves the transport of molecules between the cell’s nucleus (where genetic instructions are stored) and the cytoplasm (where those instructions are carried out). This flow is essential for maintaining cellular balance and ensuring proper gene expression.
A Domino Effect That Leads to Cognitive Decline
Neuroscientist Paul Coleman, one of the lead researchers, emphasized the core of their hypothesis: “This proposal focuses on the breakdown of communication between the nucleus and cytoplasm, leading to significant disruptions in gene activity.” According to the model, stress granules interfere with the transport channels, triggering a cascade of malfunctions at the genetic level.
These malfunctions may be directly responsible for the symptoms we associate with Alzheimer’s. Coleman and his team believe that the formation of tau tangles, inflammation in the brain, and the development of amyloid-beta plaques may all be secondary consequences of this primary issue.
“The diverse aspects of Alzheimer’s might originate from this single issue,” he explained.
Early Stress, Earlier Intervention
What makes this theory particularly promising is its potential for early detection. Since stress granule-related changes occur before visible symptoms emerge, this new model could open up a valuable window of opportunity for intervention.
If scientists can develop strategies to detect or prevent the abnormal persistence of stress granules, they may be able to stop Alzheimer’s before it has a chance to take hold. Environmental factors—such as air pollution, long-term inflammation, or even certain genetic mutations—may contribute to this process, highlighting areas for future research and preventive care.

Redefining the Timeline of Alzheimer’s
Dr. Coleman noted that this model adds weight to the ongoing debate about when Alzheimer’s truly begins. His team believes the cellular changes involving stress granules could be among the earliest biological signs of the disease.
Key questions remain unanswered: At what point do these changes become irreversible? When is the optimal time to intervene? And could routine screening for stress granule activity help identify at-risk individuals before memory loss begins?
The answers to these questions could transform how the disease is managed and, eventually, how it is treated.
What Are Stress Granules and Why Are They Important?
Stress granules are temporary structures that form in a cell’s cytoplasm during times of physiological stress. These stresses can come from viral infections, heat shock, toxic exposures, or nutrient shortages. The granules serve as a kind of emergency storage unit, containing messenger RNA (mRNA) and RNA-binding proteins that help regulate gene activity under pressure.
Once the stressful event passes, healthy cells break down the stress granules, releasing the mRNA back into circulation to resume normal protein synthesis. This reversible process is essential for helping the cell survive and return to normal function.
Where Things Go Wrong in Alzheimer’s
According to the Arizona State University researchers, in Alzheimer’s disease, this natural cycle of stress granule formation and dissolution goes off track. Rather than dissolving, the granules remain in the cell, where they start to interfere with fundamental operations.
The most serious disruption seems to occur in nucleocytoplasmic transport—the process that manages the flow of molecules between the nucleus and the rest of the cell. This process ensures that the genetic code is properly interpreted and that proteins are made accurately and on time.

The Role of the Nuclear Pore Complex
At the heart of this transport system is the nuclear pore complex (NPC), a highly regulated gateway embedded in the membrane of the cell’s nucleus. Molecules must pass through this portal to carry genetic messages in and out.
Transport proteins guide this movement with precision, but persistent stress granules may interfere with this delicate exchange, contributing to cellular dysfunction.
How This Triggers Alzheimer’s Symptoms
The proposed chain reaction begins with stress granules and leads to the wide range of symptoms seen in Alzheimer’s:
A New Frontier in Early Diagnosis and Prevention
The most promising implication of this new theory is that stress granules could be used as an early biomarker of Alzheimer’s—possibly years before symptoms emerge. If clinicians can find a way to detect the abnormal persistence of stress granules, they may be able to diagnose the disease much earlier than current methods allow.
Therapeutic strategies could then focus on dissolving or preventing the buildup of stress granules altogether. This might involve developing new medications or interventions that specifically target the cellular stress response.
Where the Research Goes From Here
Although this model is still in its early stages, it offers a more unified explanation of Alzheimer’s and opens the door for new avenues of exploration. Future research will likely aim to:
A Simplified, Hopeful View of a Complex Disease
Alzheimer’s is often described as a mysterious and multifaceted condition—but this new theory introduces a powerful idea: perhaps its complexity stems from a single cellular failure. If stress granules truly lie at the core of the disease, then focusing research on this early-stage process could bring us closer to more effective treatments—or even prevention.
While many questions remain, the Arizona State University team’s work represents a major step forward. By highlighting the first domino in the chain reaction that leads to Alzheimer’s, they’ve given researchers and clinicians a new place to start—and perhaps, a reason for hope.

If you are one of those people who prefer their eggs hard-boiled, you have certainly noticed that green color ring around the yolk.



Unlike traditional sodas loaded with added sugar and artificial ingredients, Milaf Cola delivers a refreshingly sweet experience using only the natural sugars found in dates.


It reminds us that the story of Jesus wasn’t just passed down through scripture - it also traveled through languages, translations, and centuries of human culture.

Discovering a precise, side‑effect‑free neural switch for anxiety is a major scientific breakthrough.
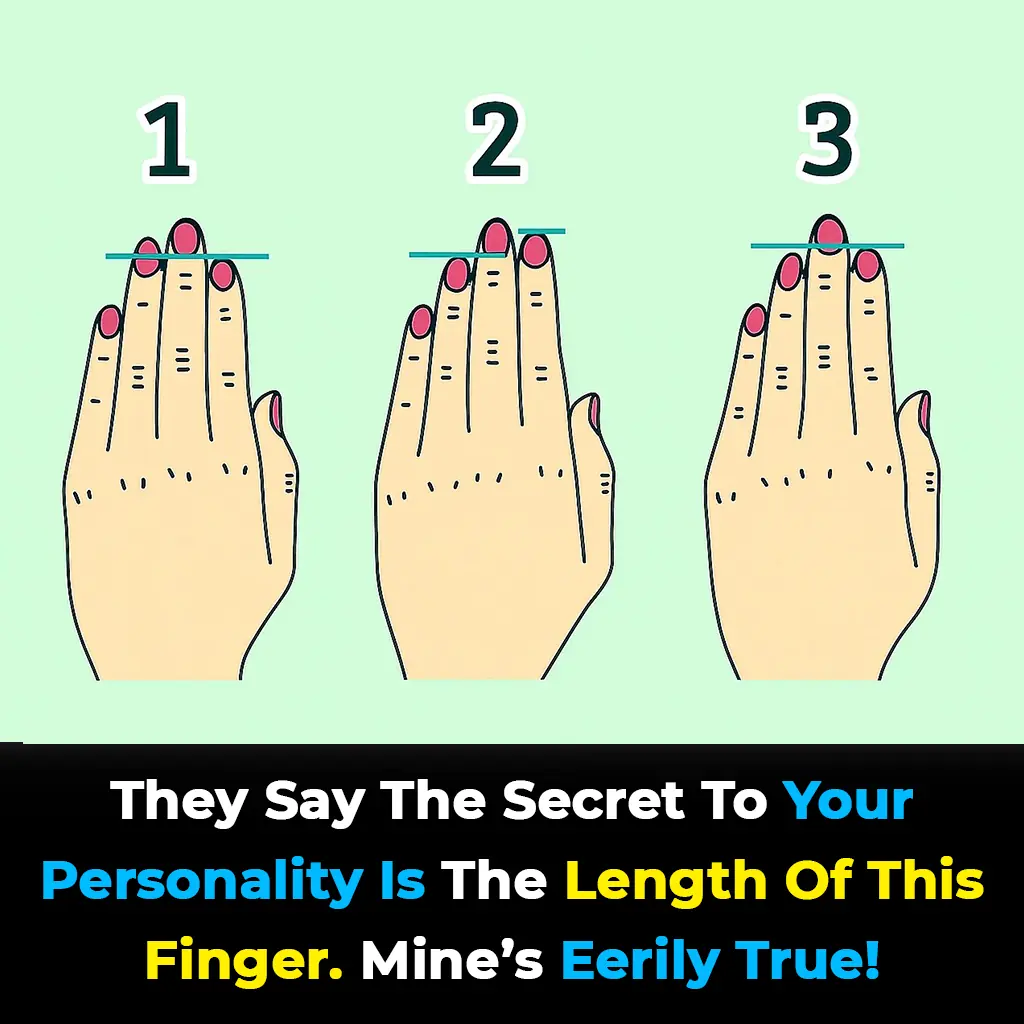
Some believe your finger length can reveal key aspects of your personality through a theory called the digit ratio. Though not scientifically proven, it links finger proportions to traits like confidence, empathy, and risk-taking.

While flight attendants are being polite, there is another reason why they say 'hello' as you board

If you see a flight attendant seated with their hands tucked underneath them during landing, remember—it’s not just a quirky habit.









If you are one of those people who prefer their eggs hard-boiled, you have certainly noticed that green color ring around the yolk.









Unlike traditional sodas loaded with added sugar and artificial ingredients, Milaf Cola delivers a refreshingly sweet experience using only the natural sugars found in dates.

The embryo the Pierces adopted was originally created through in vitro fertilization (IVF) back in 1994, making it older than many of today’s prospective parents themselves, according to MIT Technology Review.





Passing gas up to 25 times a day can be considered normal - but when you notice a sudden increase, especially if it's paired with discomfort or other symptoms, it's time to tune in.

It reminds us that the story of Jesus wasn’t just passed down through scripture - it also traveled through languages, translations, and centuries of human culture.