
Shocking findings of new trial on Ozempic alternative pushes it closer to approval
 New Daily Weight Loss Pill Could Rival Ozempic With Impressive Trial Results
New Daily Weight Loss Pill Could Rival Ozempic With Impressive Trial Results
A new weight loss medication currently being trialed as a once-daily pill is showing tremendous promise—and it might soon compete directly with popular injectable drugs like Ozempic.
An estimated one in eight Americans have tried GLP-1 receptor agonist medications such as Ozempic, primarily prescribed for managing type 2 diabetes. While effective, these drugs can come with unpleasant side effects like nausea, fatigue, and digestive discomfort.
It's worth noting that Ozempic itself isn’t FDA-approved for weight loss, even though it’s widely used off-label for that purpose. However, other GLP-1-based medications, including semaglutide (Wegovy) and liraglutide (Saxenda), are officially approved for treating obesity.
Now, Eli Lilly, a pharmaceutical giant, may have made a breakthrough that changes the game entirely. They’ve developed Orforglipron, an experimental drug that belongs to the GLP-1 receptor agonist class but comes in the form of a simple pill.
Unlike injectable options like Ozempic, Orforglipron is taken orally once a day—without the need for food or even water, according to Verywell Health. That makes it significantly more convenient than alternatives like Rybelsus, the current GLP-1 pill on the market, which requires strict timing around meals and can cause significant nausea in some users.
What really has scientists and medical professionals excited, though, are the early clinical trial results.
In a 40-week study, Orforglipron was shown to reduce participants' A1C levels—a critical marker of long-term blood sugar control—by up to 1.6%. Even more impressive: among those on the highest dose (36 mg), nearly two-thirds achieved blood sugar levels below the threshold for diabetes.
And it doesn’t stop there.
Participants on the highest dose also lost an average of 8% of their body weight, or around 16 pounds. Remarkably, this weight loss occurred before the participants had even reached a plateau, meaning further reductions may have been possible had the trial continued beyond 40 weeks.
Another major advantage? Convenience. With Orforglipron, users can skip the weekly injections, potentially making treatment adherence easier and less invasive.
Side effects so far have been mild, mostly limited to gastrointestinal discomfort—a common issue across the GLP-1 drug class. More importantly, Orforglipron has shown no signs of liver toxicity, a serious concern that led Pfizer to pull the plug on its own GLP-1 pill candidate.
So what’s next?
Eli Lilly is aiming to secure FDA approval for Orforglipron as a weight-loss treatment later this year. Approval for diabetes treatment is projected to follow by 2026.
If approved, Orforglipron could become a market-shifting alternative to injectables like Ozempic and Wegovy, offering the same benefits with a much simpler and more user-friendly approach.
With rising obesity rates and millions seeking more accessible treatment options, this could be the pill that changes everything.
Is this the beginning of a new era in weight management?
It certainly looks that way.
News in the same category

🧠 8 Strange (But Real) Signs Your Body Is Begging for More Vitamin B12 – Don’t Ignore These Red Flags

How To Identify Skin Tags and When To Remove Them
14 Visible Signs of Cancer Most Women Ignore

Reducing Prostate Discomfort Naturally with a Tomato and Garlic Drink

Never Throw Away the Avocado Seed Again — Here’s Why

Guava Leaves Benefits: The Underrated Natural Remedy You Shouldn’t Ignore 🍃💪

Herbal Tea for Swollen Legs: Natural Diuretic & Anti-Inflammatory Recipe, How to Use It, and Precautions
Avocado Seed Benefits: The Overlooked Natural Remedy for Joint and Back Pain
How Garlic and Lemon Can Gently Support Your Eye Comfort and Vision Wellness

The Overlooked Tree With Powerful Health Benefits

Discover How Incorporating Fresh Parsley into Your Daily Routine Can Support Knee Joint Comfort and Mobility Naturally

Woman reveals 3 overlooked symptoms before her stage 4 cancer diagnosis at 28
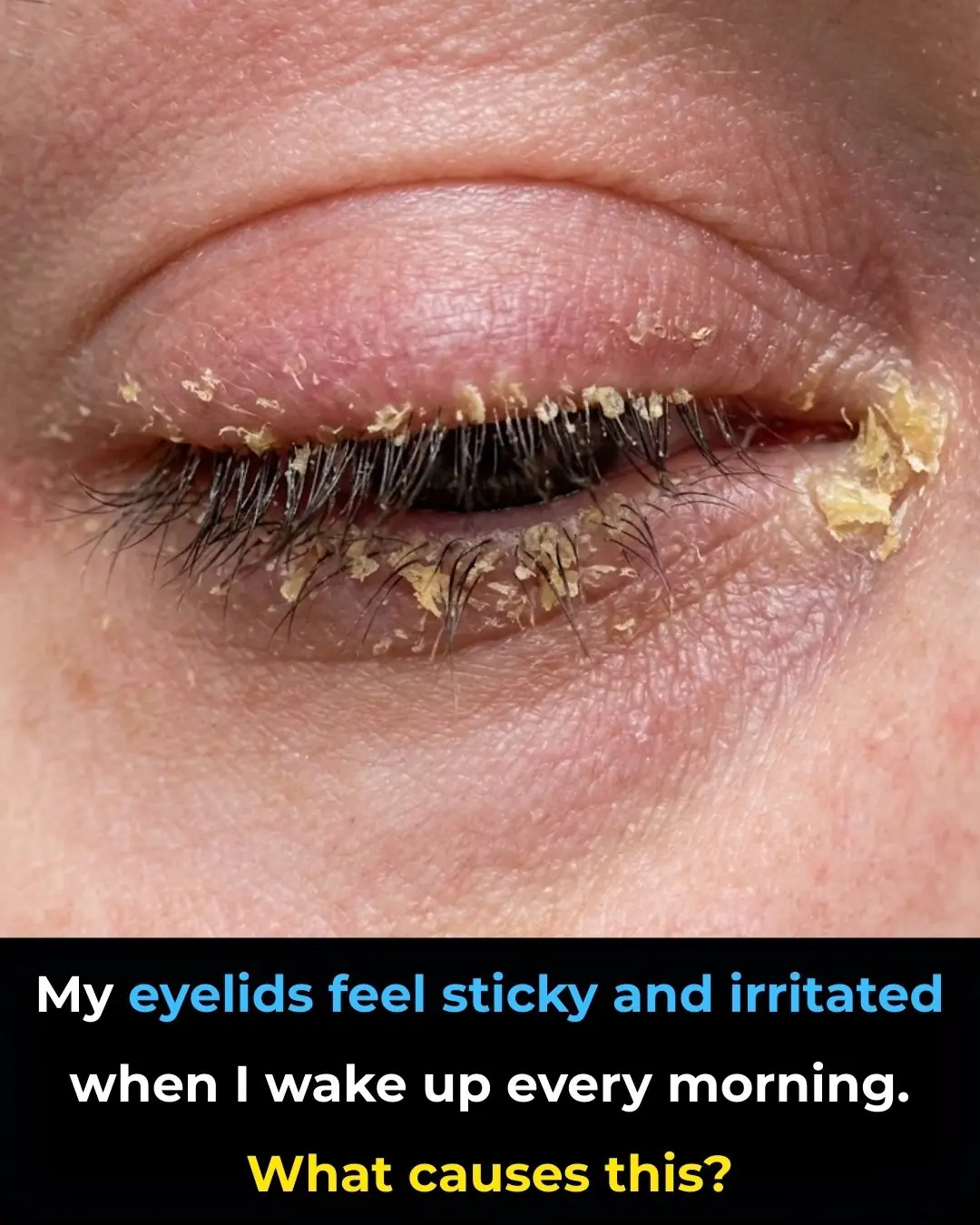
My eyelids feel sticky and irritated when I wake up every morning. What causes this?

One Spoon a Day for Stronger Vision – A Simple Daily Habit to Support Eye Comfort

A Gentle Herbal Infusion to Support Blood Sugar, Cholesterol & Circulation Naturally
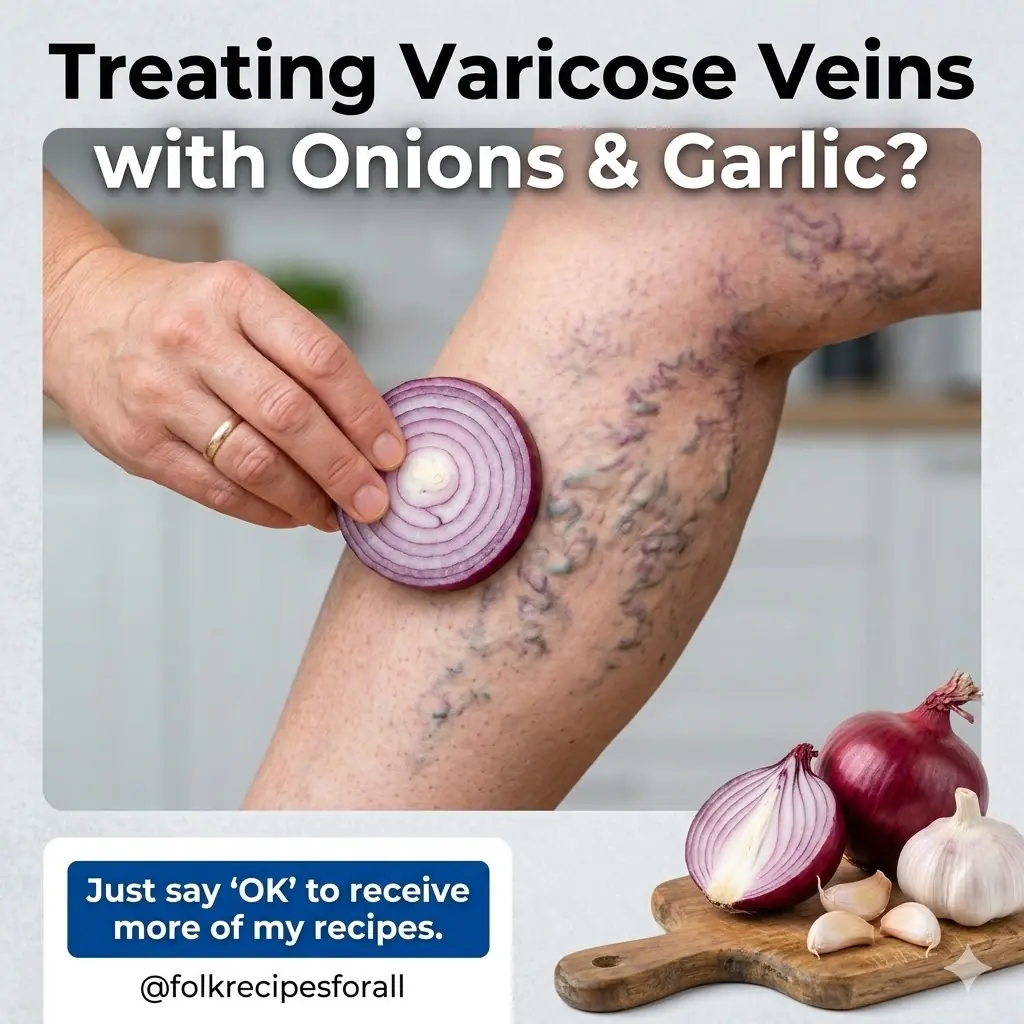
Home Remedies for Varicose Veins with Onion, Garlic, and Apple Cider Vinegar
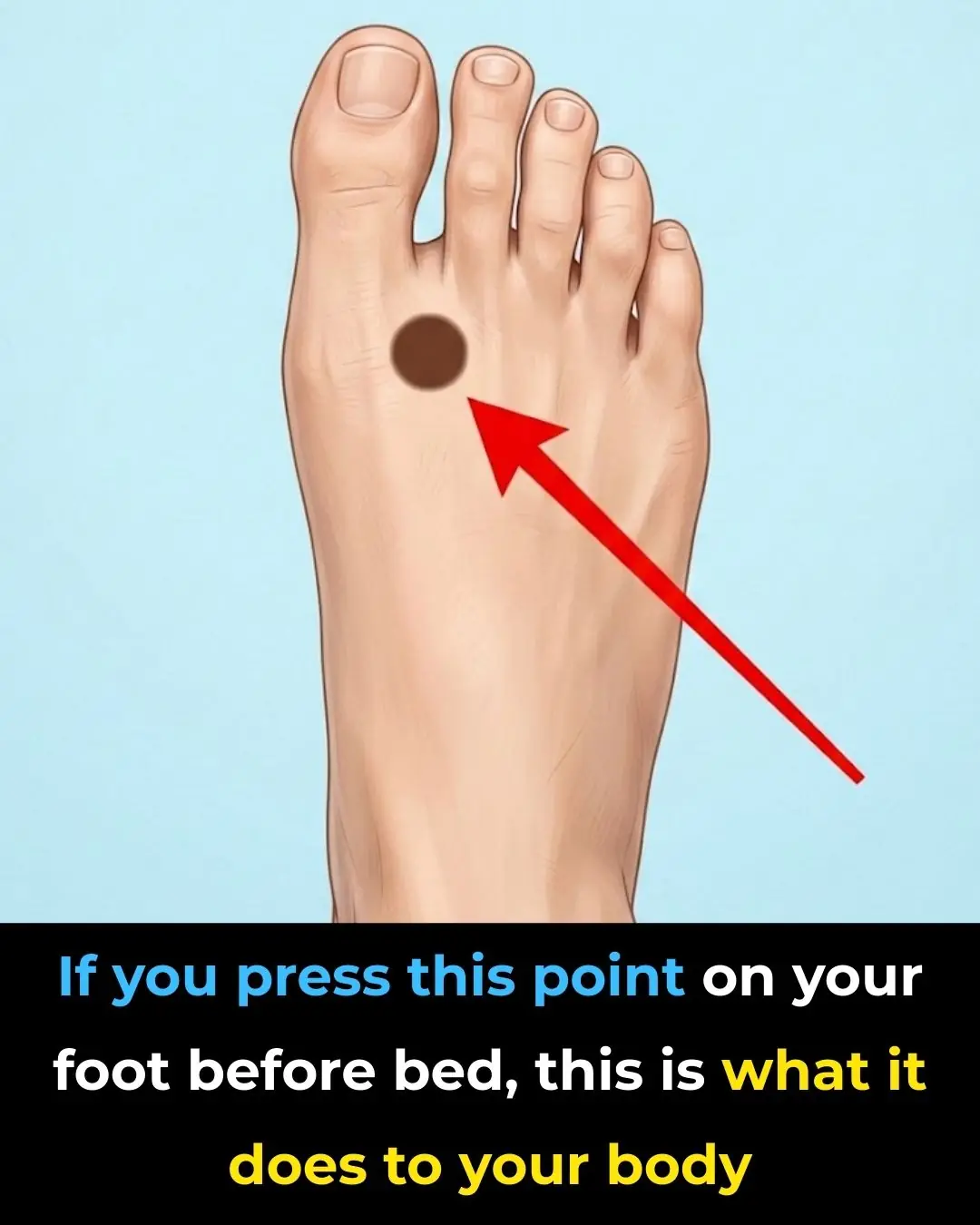
Press This Point on Your Feet Before Bed
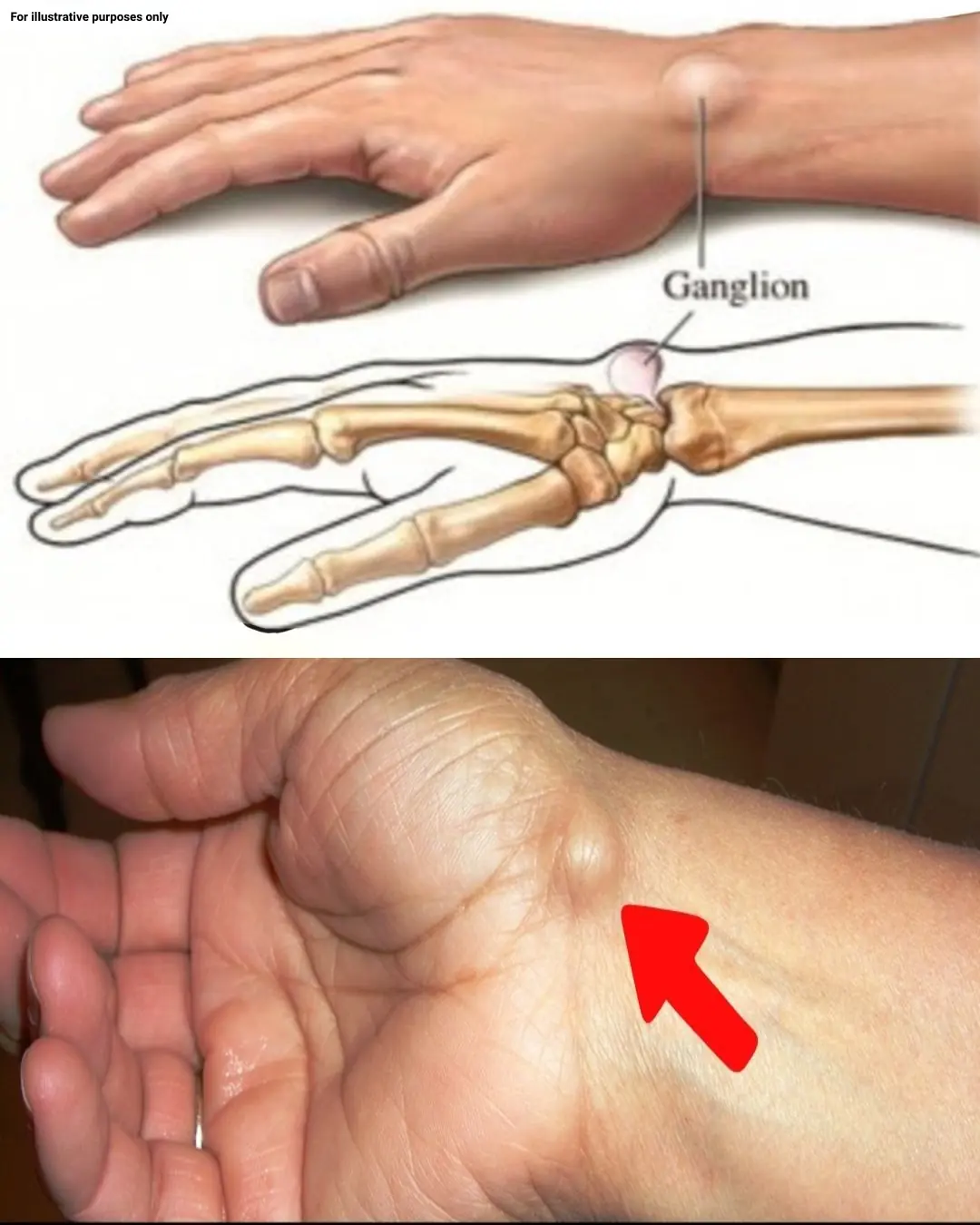
Noticing a Lump on Your Hand
More Than a Moment: Understanding the Layers of Intimacy
News Post

Everything You Need To Know About Nail Pitting

🧠 8 Strange (But Real) Signs Your Body Is Begging for More Vitamin B12 – Don’t Ignore These Red Flags

How To Identify Skin Tags and When To Remove Them

14 Visible Signs of Cancer Most Women Ignore

Reducing Prostate Discomfort Naturally with a Tomato and Garlic Drink

Never Throw Away the Avocado Seed Again — Here’s Why

Guava Leaves Benefits: The Underrated Natural Remedy You Shouldn’t Ignore 🍃💪

Herbal Tea for Swollen Legs: Natural Diuretic & Anti-Inflammatory Recipe, How to Use It, and Precautions

Avocado Seed Benefits: The Overlooked Natural Remedy for Joint and Back Pain

How Garlic and Lemon Can Gently Support Your Eye Comfort and Vision Wellness

Was He Really Jesus?

Pumpkin Seeds

The Overlooked Tree With Powerful Health Benefits

Discover How Incorporating Fresh Parsley into Your Daily Routine Can Support Knee Joint Comfort and Mobility Naturally

Woman reveals 3 overlooked symptoms before her stage 4 cancer diagnosis at 28

The Funeral Secret

My eyelids feel sticky and irritated when I wake up every morning. What causes this?

My feet feel hot and burning when I lie down at night, even though they’re cold to touch. What’s going on?

One Spoon a Day for Stronger Vision – A Simple Daily Habit to Support Eye Comfort
