
Fenbendazole and Unexpected Cancer Remission: Insights from a 2024 Case Report
Cancer treatment failure remains a devastating reality for many patients with advanced or treatment-resistant malignancies. When standard therapies such as surgery, chemotherapy, immunotherapy, and hormonal therapy no longer halt disease progression, therapeutic options become extremely limited. In this context, isolated reports of unexpected remission—particularly involving repurposed or unconventional agents—attract significant scientific interest, not as proof of efficacy, but as potential signals warranting deeper investigation. A 2024 case report published in Exploratory Research and Hypothesis in Medicine describes three such unusual cases involving fenbendazole, a benzimidazole antiparasitic drug commonly used in veterinary medicine.
The report was authored by a clinical team from Kyungpook National University Hospital and documented three individual patients with advanced, refractory cancers. The patients included one individual with stage IV metastatic breast cancer, one with recurrent malignant melanoma, and one with castration-resistant prostate cancer. All three had experienced continued disease progression despite receiving multiple lines of conventional treatment, including combinations of surgery, chemotherapy, immunotherapy, and hormone-based therapy. Facing limited remaining options, each patient independently chose to self-administer veterinary-grade fenbendazole outside of standard medical protocols.
According to the authors, all three patients subsequently achieved complete or near-complete remission, as confirmed by radiological imaging. Remarkably, these responses were sustained over follow-up periods ranging from 11 months to 34 months, during which no evidence of disease recurrence was observed. Equally notable was the reported absence of fenbendazole-related adverse effects in all three cases, despite prolonged use. From a clinical perspective, the durability of the responses and the consistency across three different cancer types make these observations particularly striking.
Fenbendazole belongs to the benzimidazole class of compounds, which are known to disrupt microtubule formation in parasites. This mechanism has drawn interest in oncology because microtubules are also critical for cancer cell division. Preclinical studies have suggested that benzimidazoles may inhibit tumor growth by interfering with mitosis, inducing apoptosis, altering glucose metabolism, and impairing cancer cell survival pathways. However, robust clinical evidence supporting their anti-cancer efficacy in humans is currently lacking.
The authors of the case report were careful to emphasize the limitations of their findings. Case reports, by nature, cannot establish causality. The observed remissions may have been influenced by delayed effects of prior treatments, spontaneous regression, individual tumor biology, or other unrecognized factors. Additionally, because the patients self-administered fenbendazole without standardized dosing or controlled conditions, the findings cannot be generalized or used to guide clinical practice. The use of veterinary-grade medication also raises important safety, ethical, and regulatory concerns.
Nevertheless, the report serves an important scientific purpose. Throughout medical history, unexpected clinical observations have occasionally provided the first clues leading to major therapeutic breakthroughs. The authors argue that these cases should not be interpreted as evidence that fenbendazole is a proven cancer treatment, but rather as hypothesis-generating signals that justify further investigation. Carefully designed laboratory studies, followed by controlled animal experiments and ultimately human clinical trials, would be required to determine whether fenbendazole has genuine anti-cancer activity, to clarify its mechanisms of action, and to assess its safety profile in oncology patients.
In conclusion, the 2024 case report published in Exploratory Research and Hypothesis in Medicine describes three patients with advanced, treatment-resistant cancers who experienced sustained complete or near-complete remission after self-administering fenbendazole (Exploratory Research and Hypothesis in Medicine, 2024). While these observations are intriguing, they remain anecdotal and insufficient to support clinical use. Nonetheless, they highlight the potential value of systematically exploring repurposed drugs and underscore the importance of rigorous scientific follow-up to distinguish coincidence from causality in cancer research.
News in the same category

Sodium Bicarbonate and Immune Regulation: Evidence for an Anti-Inflammatory Mechanism
Oleocanthal from Extra-Virgin Olive Oil: A Multi-Targeted Anti-Inflammatory and Anti-Cancer Compound
Quercetin as an Exercise Mimetic: Enhancing Mitochondrial Biogenesis and Physical Performance
Dual “Don’t-Eat-Me” Signals: A New Paradigm in Cancer Immune Evasion

Cinnamon and Neuroprotection: Evidence for Anti-Alzheimer’s Mechanisms

High-Dose Thiamine and Cancer Cell Metabolism: Evidence from Experimental Cell-Line Studies
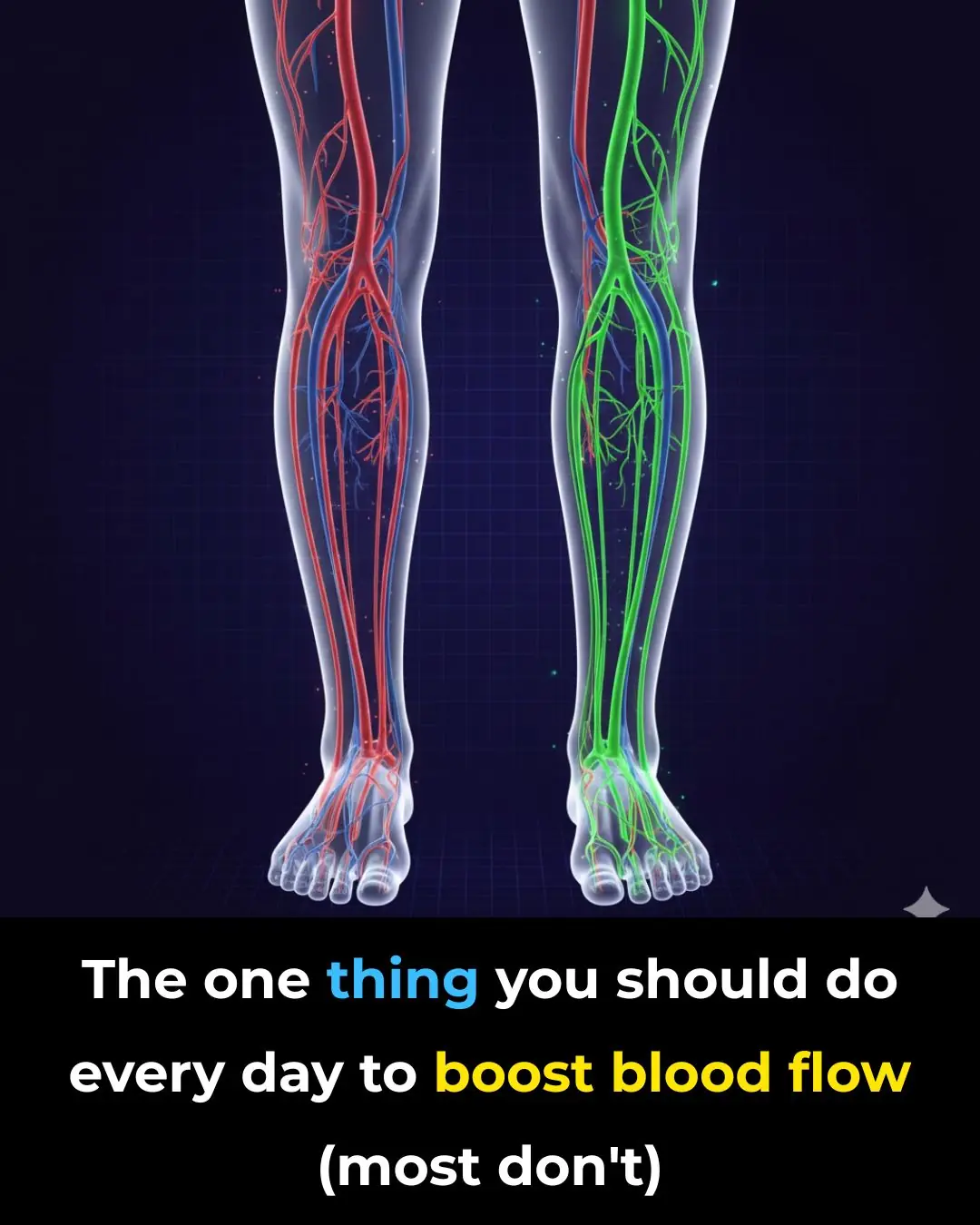
How to Improve Blood Circulation Naturally (Research Based)

The Most Effective Ways to Naturally Get Rid of Clogged Ears
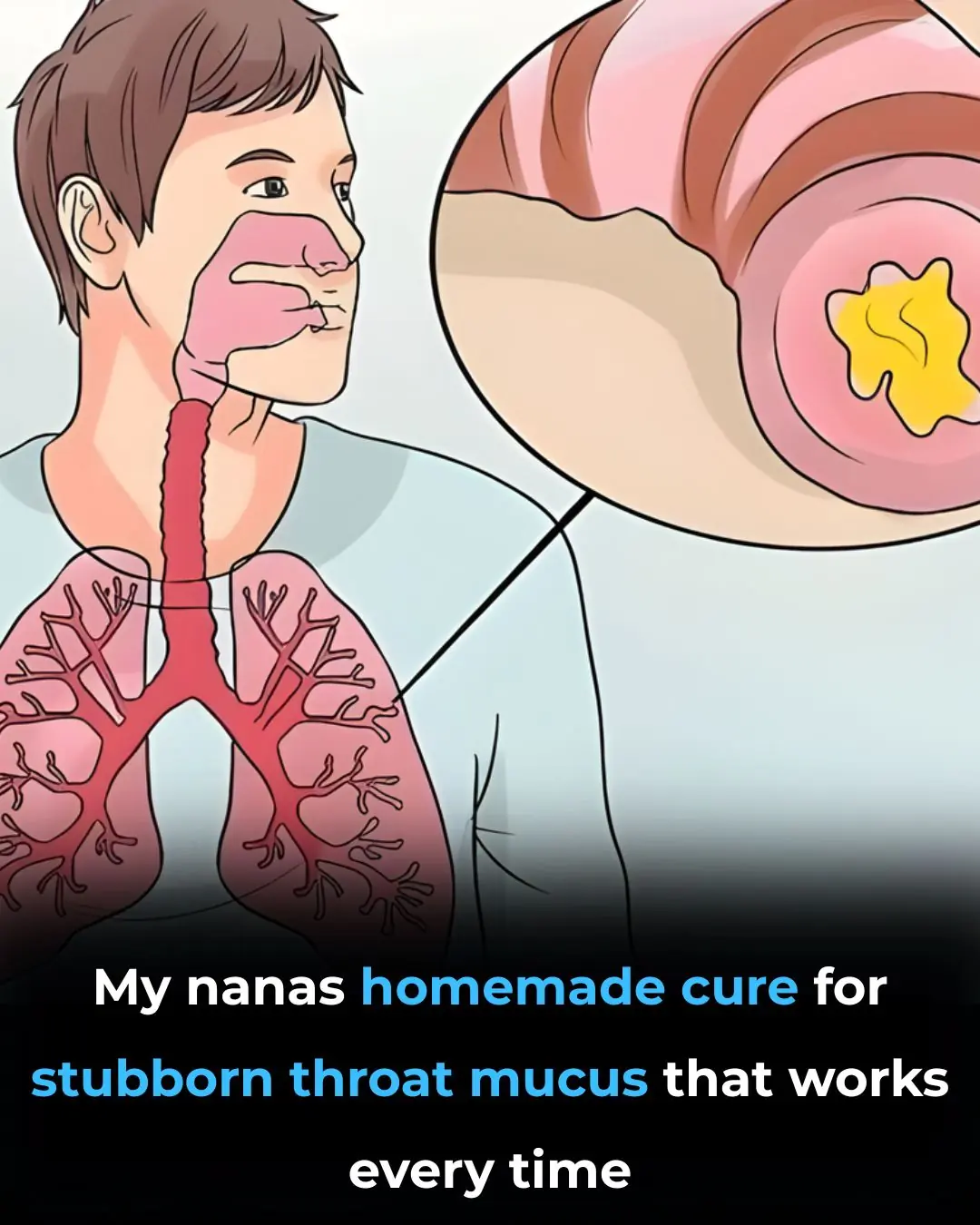
Get Rid of Throat Mucus Faster With These Home Treatments (Evidence Based)

The Most Likely Symptoms of a Gallbladder Problem (Don’t Ignore Them)
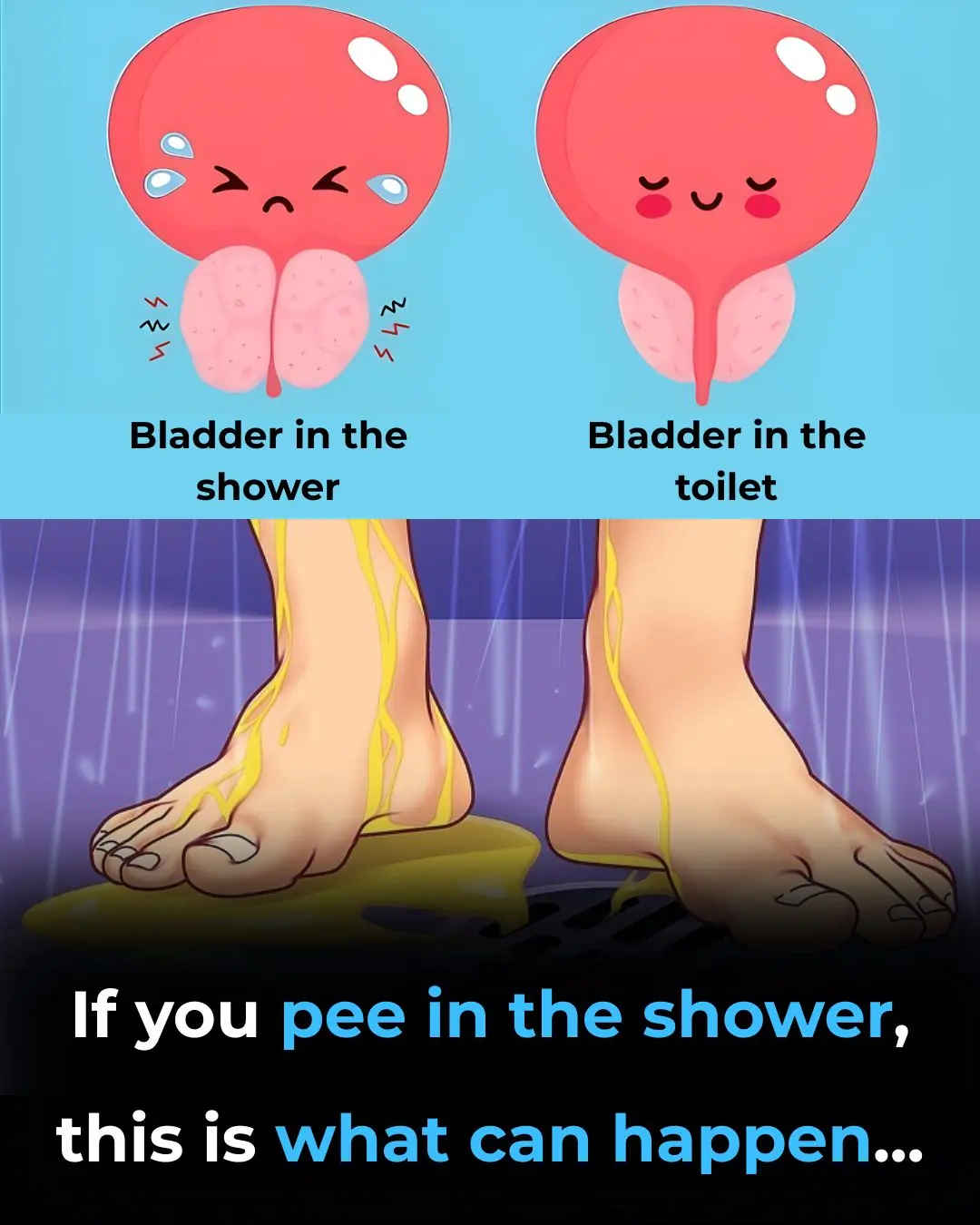
Why Hearing Running Water Makes You Suddenly Need to Pee

The strange phenomenon of sleep paralysis: When the body stops listening

Surprising Health Benefits of Purslane (Portulaca oleracea)

Ageratum Conyzoides: A Valuable Herb with Many Health Benefits

The man died of colorectal cancer, his family broke down in tears: "He ate healthily but liked to do three things."

Women Who Regularly Eat These 4 Foods Can Prevent Premature Graying, Keeping Hair Dark and Shiny Even at 60

The Root that Helps the Liver Detoxify and Effectively Reduce Inflammation
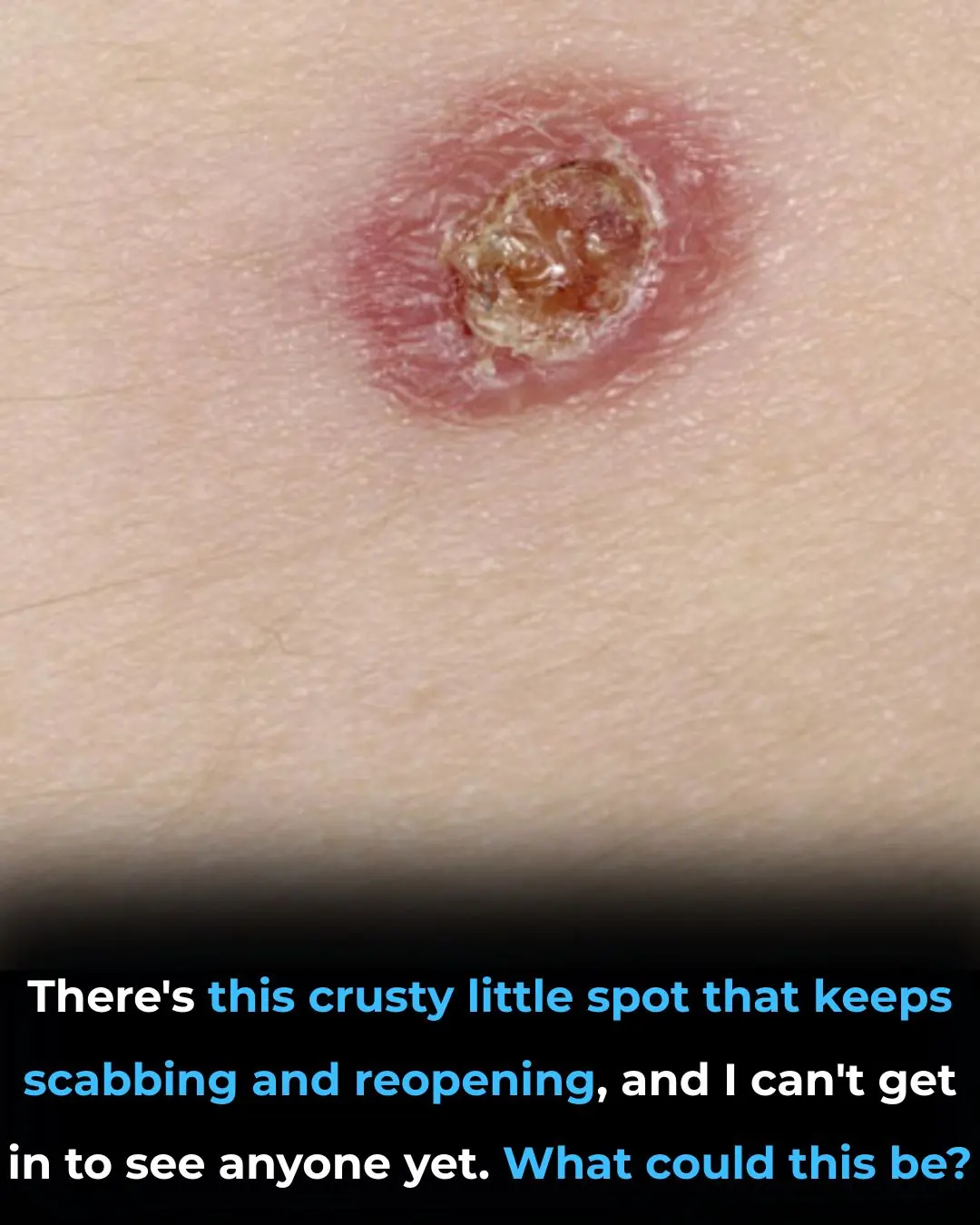
There’s this crusty little spot that keeps scabbing and reopening, and I can’t get in to see anyone yet. What could this be?
News Post

Nighttime Olfactory Enrichment and Cognitive Enhancement in Older Adults

Sodium Bicarbonate and Immune Regulation: Evidence for an Anti-Inflammatory Mechanism

Oleocanthal from Extra-Virgin Olive Oil: A Multi-Targeted Anti-Inflammatory and Anti-Cancer Compound

Quercetin as an Exercise Mimetic: Enhancing Mitochondrial Biogenesis and Physical Performance

Dual “Don’t-Eat-Me” Signals: A New Paradigm in Cancer Immune Evasion

Cinnamon and Neuroprotection: Evidence for Anti-Alzheimer’s Mechanisms

High-Dose Thiamine and Cancer Cell Metabolism: Evidence from Experimental Cell-Line Studies
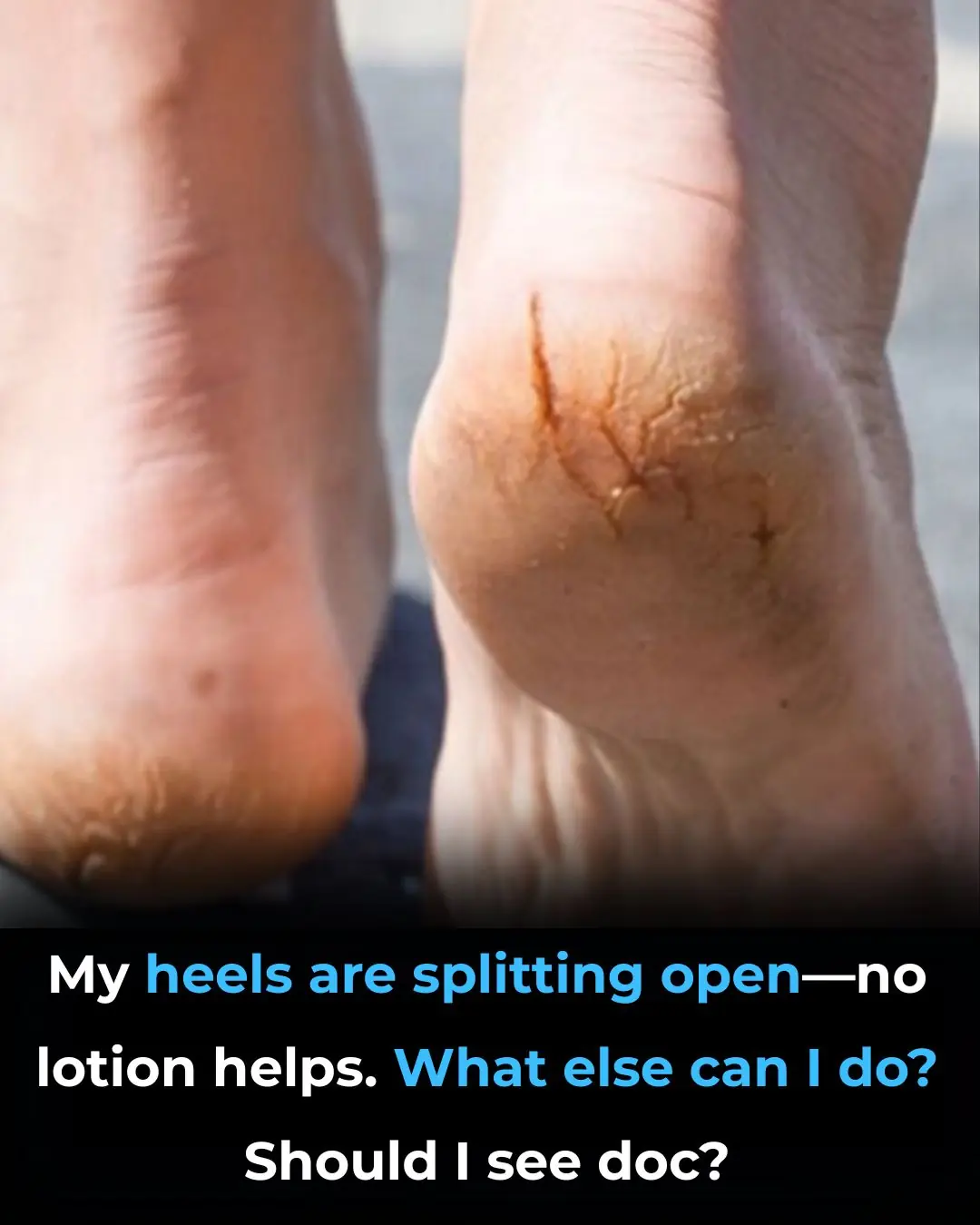
My heels are splitting open—no lotion helps. What else can I do? Should I see doc?
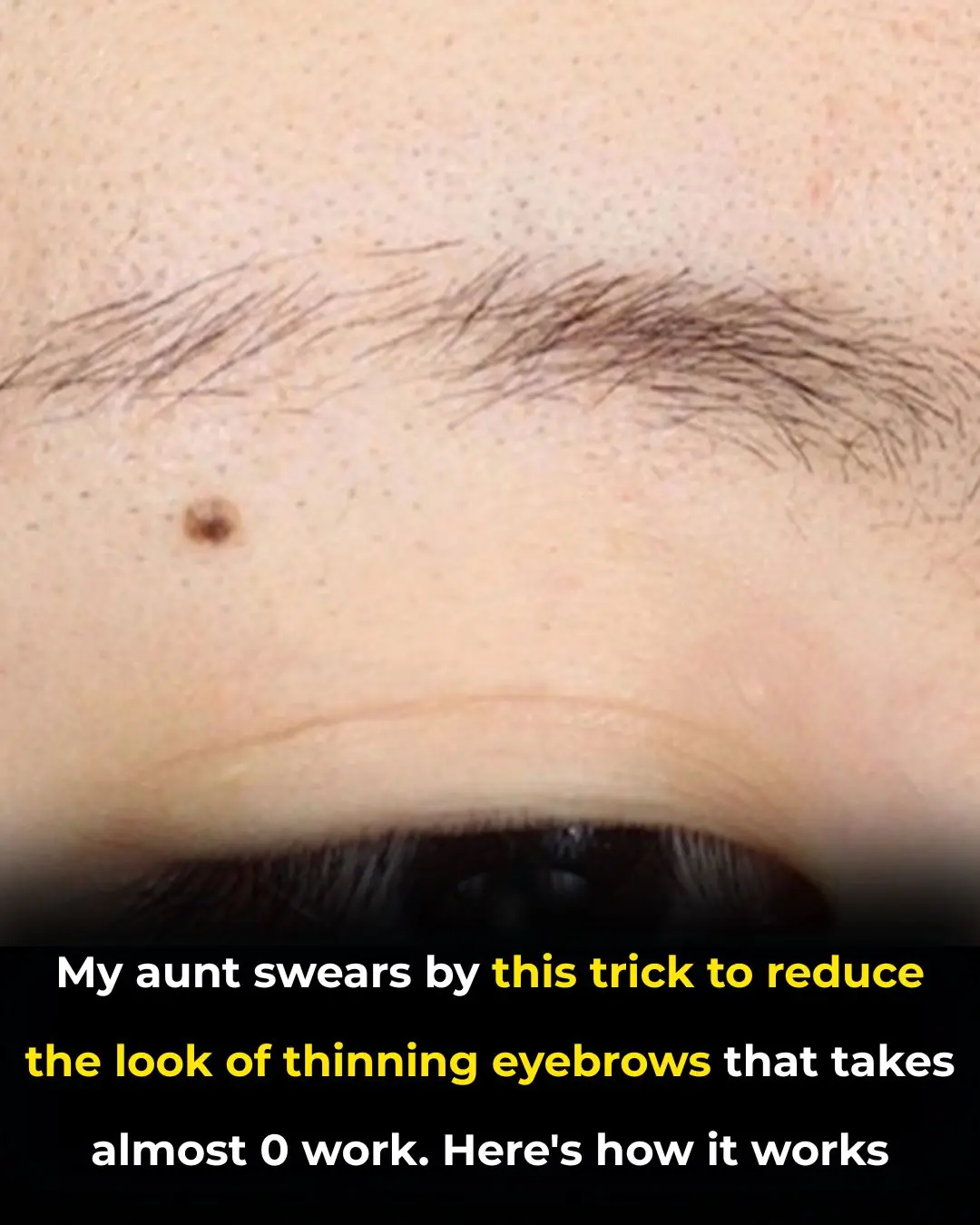
My aunt swears by this trick to reduce the look of thinning eyebrows that takes almost 0 work. Here's how it works
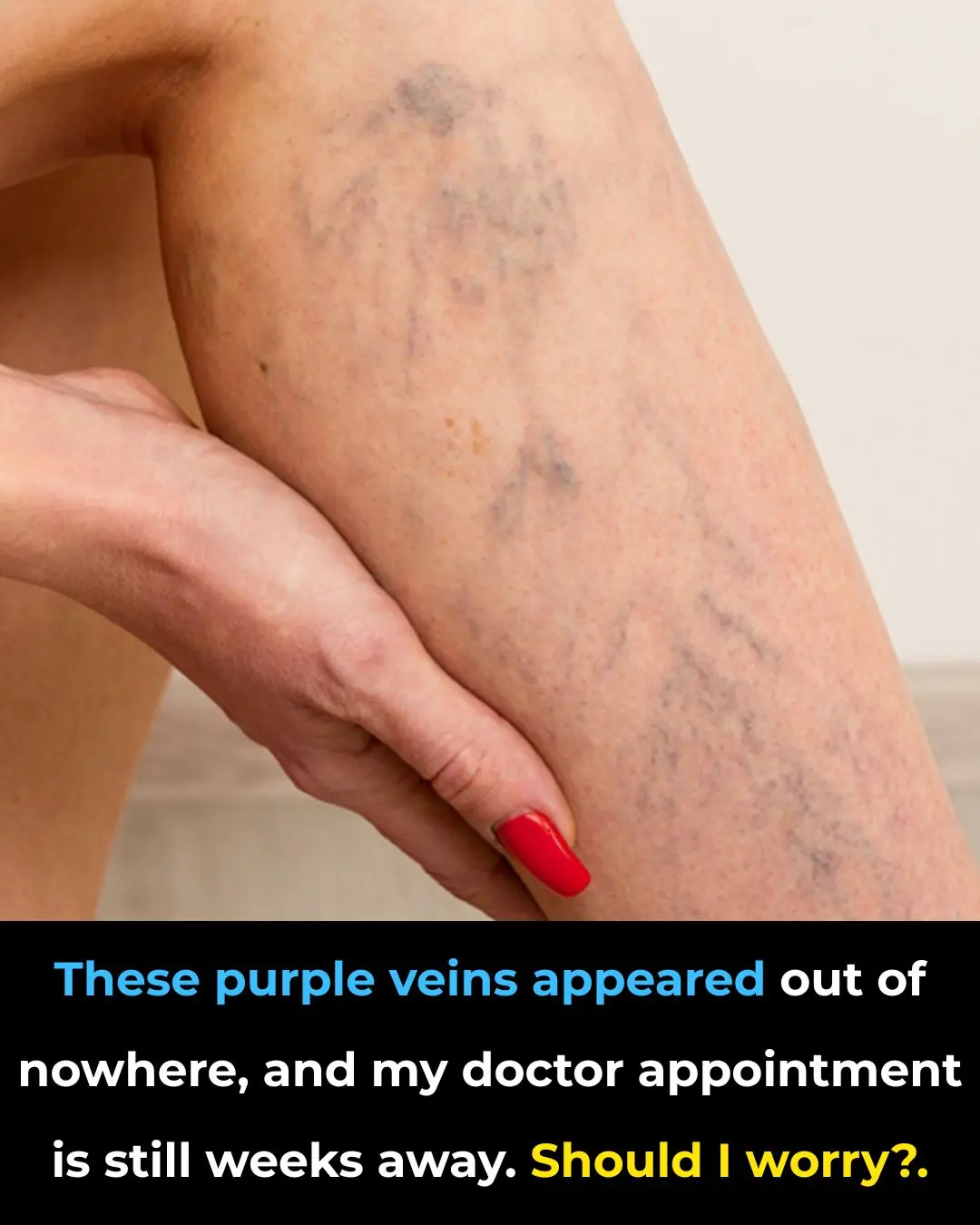
These purple veins appeared out of nowhere, and my doctor appointment is still weeks away. Should I worry?

Brown flat spots keep showing up on the back of my hands. Doctor appt is forever away. What should I do?

How to Improve Blood Circulation Naturally (Research Based)

The Most Effective Ways to Naturally Get Rid of Clogged Ears

Get Rid of Throat Mucus Faster With These Home Treatments (Evidence Based)

The Most Likely Symptoms of a Gallbladder Problem (Don’t Ignore Them)

If A Woman Says These 6 Things Regularly, She’s Way Smarter Than You Even Realize

11 Clever Phrases Smart People Use to End Pointless Arguments

Mopping the floor with this ingredient will make it sparkling clean, like new, and dust-free for a whole week.
