
Health Alert: Contaminated DermaRite Products Recalled Across U.S. and Puerto Rico
DermaRite Industries has issued a nationwide recall of multiple antiseptic and antimicrobial soap products after laboratory tests detected contamination with Burkholderia cepacia complex (Bcc). This group of bacteria, commonly present in soil and water, is known for its resistance to many antibiotics, making infections difficult to treat and particularly dangerous for individuals with compromised immune systems, chronic lung conditions, or open wounds. The recall underscores the ongoing importance of strict quality control in personal care and healthcare products.
The affected products include widely distributed brands such as DermaKleen, DermaSarra, KleenFoam, and PeriGiene, which were available throughout the United States and Puerto Rico. According to the Centers for Disease Control and Prevention (CDC), healthy individuals may only experience mild, localized infections, such as minor skin irritation. However, vulnerable populations—including the elderly, hospitalized patients, and those with weakened immune defenses—face serious complications such as bloodstream infections, pneumonia, and life-threatening sepsis.
DermaRite has urged all customers to immediately check their inventory, safely dispose of any affected products, and report any adverse reactions to the U.S. Food and Drug Administration (FDA). Although no illnesses have been reported so far, the company emphasized the gravity of the situation and reaffirmed its commitment to preventing potential public health hazards. The recall serves as a reminder of the risks associated with contaminated personal care products and the critical role of regulatory oversight in protecting consumers.
Experts note that Burkholderia cepacia complex is a particularly concerning contaminant because it can survive in a variety of environments, including moist solutions and healthcare products, and can remain dormant yet infectious for extended periods. Healthcare providers and consumers are advised to exercise caution and follow recall instructions closely to minimize any risk of infection.
This recall highlights the broader need for vigilance in manufacturing and monitoring antiseptic and antimicrobial products. With the increasing prevalence of antibiotic-resistant bacteria, even routine hygiene products can pose unexpected health threats, reinforcing the necessity for robust safety testing, timely reporting, and transparent communication with the public.
News in the same category

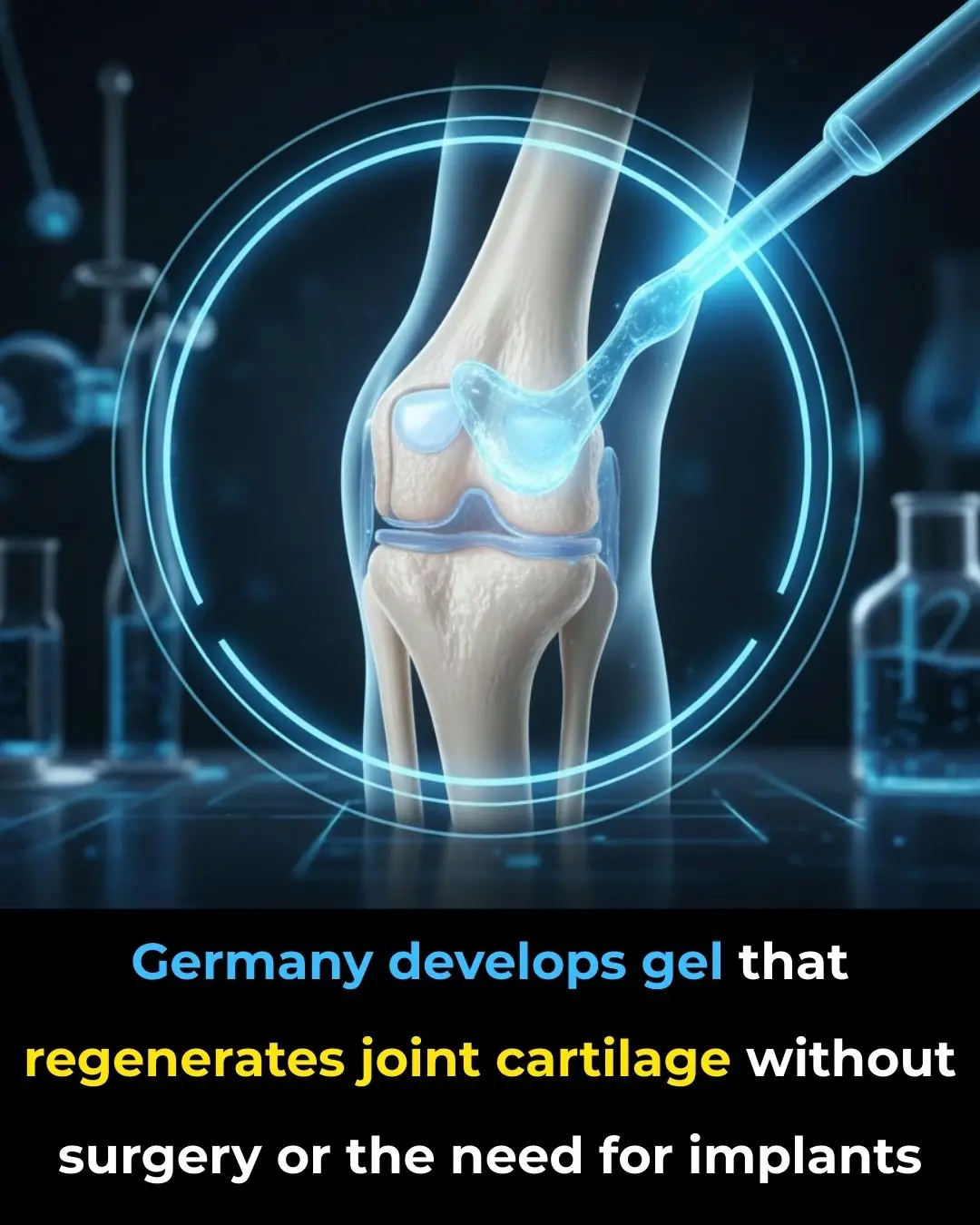
Revolutionary Gel from Germany Offers Non-Surgical Solution for Cartilage Regeneration

From Playground to Graduation: The Enduring Power of Childhood Friendship

The Spruce Pets – Creative ways to feature cats and dogs in weddings.

The Water Man of Tsavo: A Hero's Mission to Save Wildlife from Drought

Better Sleep, Healthier Spine: Why You Should Avoid Stomach Sleeping

Plumbing Mayhem on Brown Friday: How Holiday Feasts Overload Pipes

Into the Darkness: A Bioluminescent Jellyfish Illuminates the Deep Ocean

Germany’s 95% Renewable Power Day: Progress, Challenges, and Lessons for the Future

The Rower Who Chose Humanity: How Bobby Pearce Made History in Amsterdam 1928

The Secret Trick That Makes Tofu Taste Better
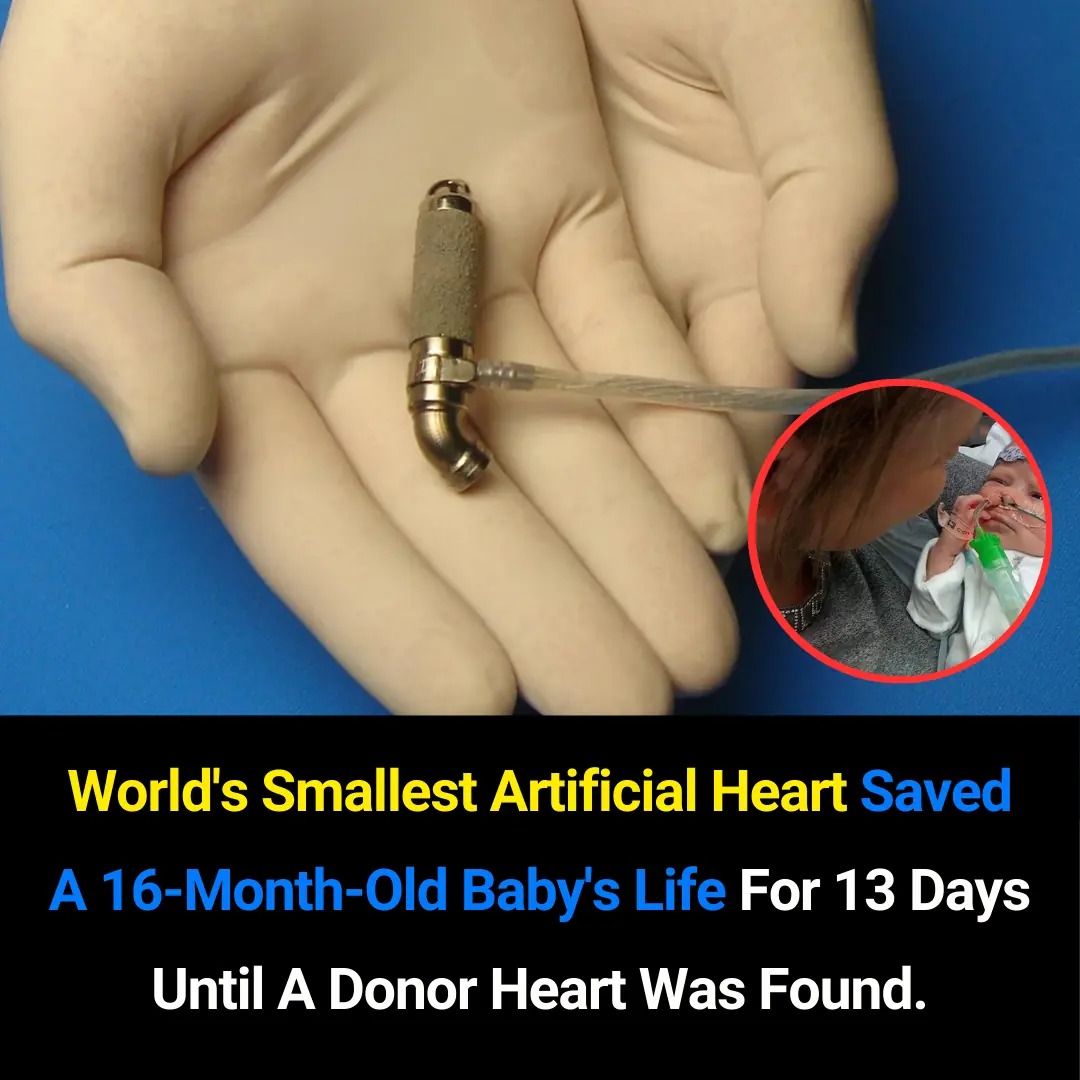
The World’s Smallest Artificial Heart: A Life-Saving Breakthrough for a 16-Month-Old Baby
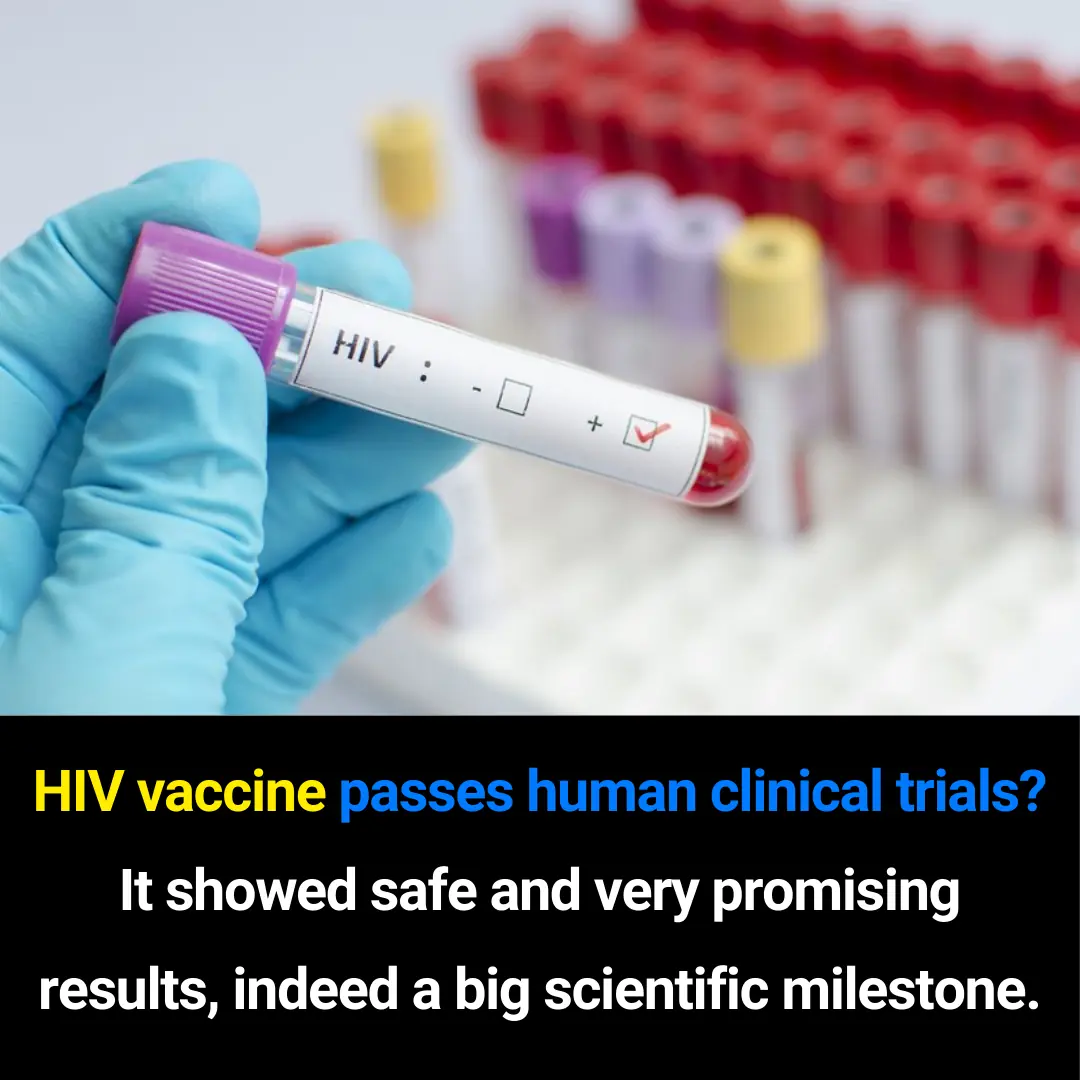
A Breakthrough in the Global Search for an HIV Vaccine: From Impossible to Truly Hopeful

Explore Canada from Coast to Coast by Train for Just $558 🇨🇦: A 3,946-Mile Adventure Through Stunning Landscapes

Golden Snub-Nosed Monkeys: A Glimpse of Wild Beauty in the Misty Mountains of China

Understanding the Extended Recovery Journey After Pregnancy

4 Things You Should Never Throw Away After a Funeral — And Why They Matter More Than You Realize

After Almost a Decade, Tennessee Zoo Celebrates Arrival of Newborn Gorilla

The Tiny Bacterium That Turns Toxic Metal Into Pure 24-Karat Gold
News Post

A Forgotten Car’s Journey: Rediscovered, Remembered, and Recycled

Revolutionary Gel from Germany Offers Non-Surgical Solution for Cartilage Regeneration

From Playground to Graduation: The Enduring Power of Childhood Friendship

The Spruce Pets – Creative ways to feature cats and dogs in weddings.

The Water Man of Tsavo: A Hero's Mission to Save Wildlife from Drought

There are two round holes on the plug, and their magical use is little known.

Better Sleep, Healthier Spine: Why You Should Avoid Stomach Sleeping

Plumbing Mayhem on Brown Friday: How Holiday Feasts Overload Pipes

Don’t Pour Hot Water Into a Clogged Sink — Do This Instead for a Quick Fix and Fresh Smell

Rare Orange Shark With Ghostly White Eyes Captured in First-of-Its-Kind Sighting

Panic Attacks And Anxiety Linked To Low Vitamin B6 And Iron levels

The Science of Rare Steak Versus Rare Chicken

Into the Darkness: A Bioluminescent Jellyfish Illuminates the Deep Ocean

Scientists Discovered How to Kill Prostate Cancer Cells Without Harming Healthy Tissue — Here’s the Breakthrough

The effortless daily trick people use to double their potassium

Germany’s 95% Renewable Power Day: Progress, Challenges, and Lessons for the Future

The Rower Who Chose Humanity: How Bobby Pearce Made History in Amsterdam 1928

Government Set to Phase Out Animal Testing and Replace It With Controversial Alternative
