
Menstrual Blood–Derived Stem Cells and Amyloid Pathology in Alzheimer’s Disease: Evidence from Preclinical Research
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by memory loss, cognitive decline, and pathological accumulation of amyloid-β (Aβ) plaques and tau tangles in the brain. Despite decades of research, disease-modifying therapies remain elusive, prompting increasing interest in regenerative and cell-based approaches. Among emerging candidates, menstrual blood–derived mesenchymal stem cells (MenSCs) have attracted attention due to their accessibility, high proliferative capacity, and potent immunomodulatory properties. A review paper titled “Menstrual Blood-Derived Stem Cells: A New Source of Stem Cells for Regenerative Medicine” synthesizes a growing body of preclinical evidence suggesting that MenSCs may reduce amyloid pathology and improve cognitive function in animal models of Alzheimer’s disease.
The review summarizes multiple in vivo studies conducted primarily in transgenic mouse models of Alzheimer’s disease, most notably APP/PS1 mice, which overexpress mutant forms of amyloid precursor protein and presenilin-1. These models develop age-dependent amyloid-β plaque accumulation in brain regions critical for learning and memory, including the hippocampus and cortex. Across the reviewed experiments, transplantation of MenSCs consistently led to significant reductions in amyloid burden. Brain analyses revealed lower levels of soluble and insoluble amyloid-β peptides, particularly Aβ40 and Aβ42, along with a marked decrease in plaque number and size in affected brain regions.
Crucially, these pathological improvements were not merely anatomical findings but were accompanied by functional benefits. Behavioral testing in MenSC-treated mice demonstrated measurable improvements in learning and memory performance, including enhanced outcomes in maze-based and object-recognition tasks. This concordance between reduced amyloid pathology and improved cognition strengthens the biological relevance of the findings, indicating that MenSC-mediated plaque reduction translated into meaningful functional recovery in animal models.
An important contribution of the review lies in its clarification of the underlying mechanisms. The authors emphasize that MenSCs do not exert their effects by differentiating into neurons or replacing lost brain cells. Instead, their therapeutic potential appears to arise from indirect, paracrine mechanisms that modify the disease environment. One major pathway involves modulation of neuroinflammation. Alzheimer’s disease is associated with chronic activation of microglia, the brain’s resident immune cells, which can adopt either neurotoxic or neuroprotective phenotypes. The reviewed studies show that MenSC transplantation promotes a microglial state more conducive to amyloid clearance, thereby enhancing the brain’s intrinsic ability to remove amyloid deposits.
In addition, MenSCs were shown to increase the activity of amyloid-degrading pathways, facilitating enzymatic breakdown and removal of Aβ peptides. The cells also secrete a range of neurotrophic and anti-inflammatory factors that support neuronal survival, reduce inflammatory signaling, and improve synaptic function. Together, these actions create a brain environment less favorable to amyloid accumulation and more supportive of cognitive processes. This multi-targeted mode of action is particularly appealing given the complex, multifactorial nature of Alzheimer’s disease.
Despite the promise of these findings, the review is explicit about their limitations. All evidence cited derives from preclinical studies conducted in animal models. While APP/PS1 mice recapitulate key aspects of amyloid pathology, they do not fully represent the complexity of human Alzheimer’s disease. Importantly, the review does not present evidence of amyloid plaque reduction or clinical benefit in humans following MenSC therapy. Questions regarding optimal dosing, delivery methods, long-term safety, immune compatibility, and efficacy in human patients remain unanswered.
In conclusion, the review published underlines that menstrual blood–derived mesenchymal stem cells represent a promising and unconventional cell source for Alzheimer’s disease research. In animal models, MenSCs reduce amyloid-β plaque burden in the hippocampus and cortex, lower Aβ40 and Aβ42 levels, and improve learning and memory through indirect mechanisms involving immune modulation and enhanced amyloid clearance. However, these results remain firmly preclinical. As emphasized by the authors, rigorous translational research and carefully designed clinical trials will be essential to determine whether the encouraging outcomes observed in mice can be replicated in humans with Alzheimer’s disease (reviewed in a peer-reviewed regenerative medicine review article).
News in the same category


Edible Mushroom Consumption and the Prevention of Subclinical Thyroid Dysfunction

Anticancer Potential of Mastic Gum Resin from Pistacia atlantica: Evidence from In Vitro Colon Cancer Models

The Effects of Raw Carrot Consumption on Blood Lipids and Intestinal Function

Off-the-Shelf CAR-NKT Cell Therapy Targeting Mesothelin: A New Strategy Against Pancreatic Cancer

The Truth About the Link Between Sugar and Cancer
Beware! 7 Neuropathy Causing Medications You Need to Know

Doctors have summarized five warning signs that a person's body typically gives in the year before death

Woman lost both kidneys before turning 30: Doctor warns of 2 habits that cause kidney failure
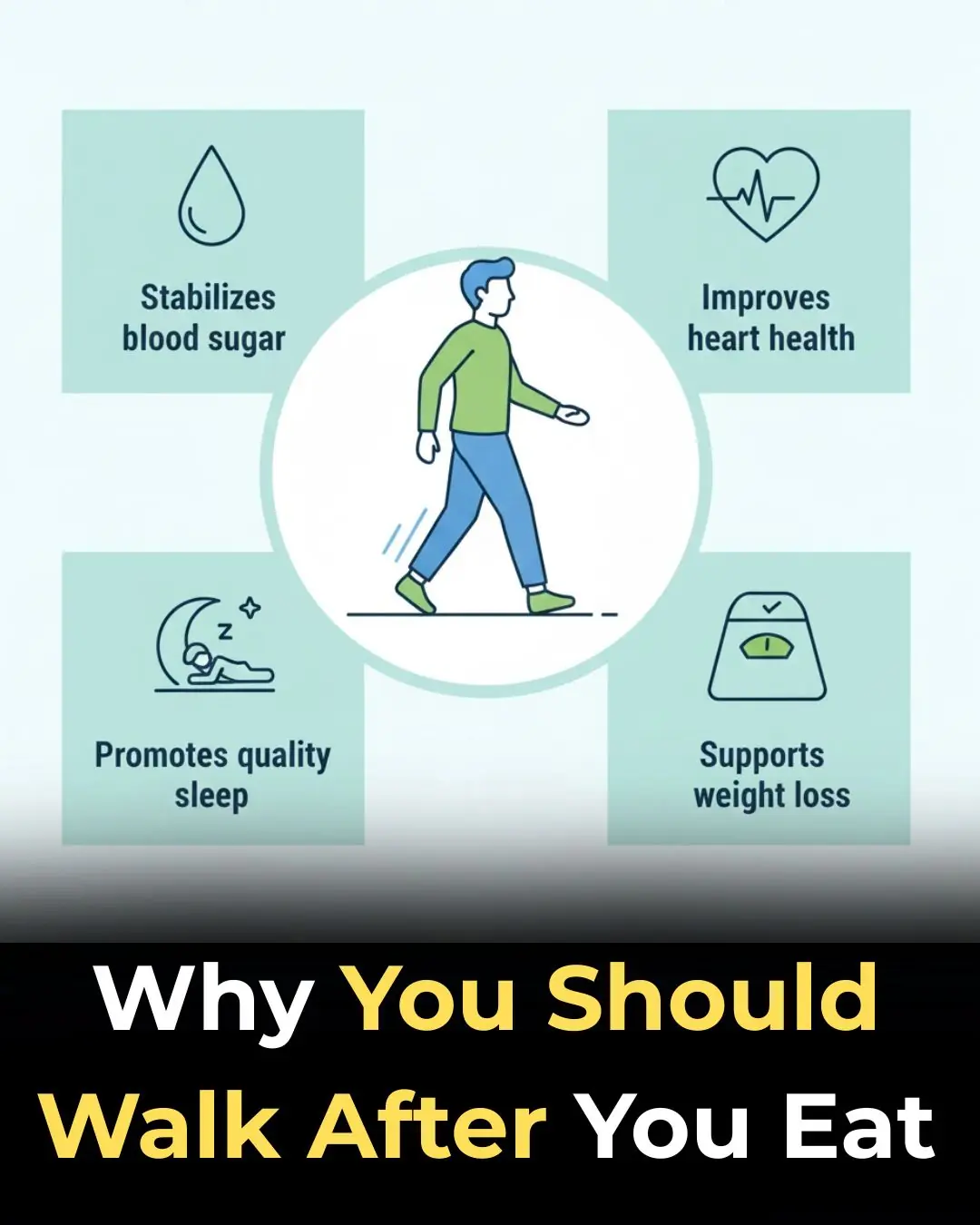
Why Walking After Eating Is So Good for You
The Best Scientifically Proven Foods to Cleanse Your Liver

5 Common Habits That Quietly Damage Your Knees
Why Hot Dogs and Processed Meat Might Be the Most Dangerous Foods of All Time
Epstein–Barr Virus May Reprogram B Cells and Drive Autoimmunity in Lupus

Oral Semaglutide Fails to Slow Cognitive Decline in Early Alzheimer’s Disease, Phase 3 Trials Show
Early Use of Glucocorticoids May Reduce Mortality in Community-Acquired Pneumonia

11 Best and Worst Foods for Boosting Metabolism

Three Ideal Times to Eat Boiled Eggs for Effective Weight Loss and Stable Blood Sugar

A 58-Year-Old Man Ate One Clove of Garlic Every Morning — His Medical Checkup Six Months Later Surprised Doctors
News Post

Household Chores and the Development of Executive Functions in Children

Edible Mushroom Consumption and the Prevention of Subclinical Thyroid Dysfunction

Anticancer Potential of Mastic Gum Resin from Pistacia atlantica: Evidence from In Vitro Colon Cancer Models

The Effects of Raw Carrot Consumption on Blood Lipids and Intestinal Function

Off-the-Shelf CAR-NKT Cell Therapy Targeting Mesothelin: A New Strategy Against Pancreatic Cancer

The Truth About the Link Between Sugar and Cancer

Most people will go their entire life without ever knowing what the drawer under the oven was actually designed for

Beware! 7 Neuropathy Causing Medications You Need to Know

Doctors have summarized five warning signs that a person's body typically gives in the year before death

Why do many people recommend squeezing lemon juice into the oil before frying?

Woman lost both kidneys before turning 30: Doctor warns of 2 habits that cause kidney failure

10 Things Women Who Have Been Heartbroken Too Many Times Do

Best Vitamins & Foods for Hair Growth

Homemade Carrot Gel for Glowing Skin & Wrinkles

Potato Toner for Face – Dark Spots, Clear Skin & Pigmentation

after an argument, my husband kicked me out and left me at a bus stop outside the city with no money.

after receiving a huge inheritance, natalya decided to see her husband’s true colors.

after the divorce, my husband threw me out the door without a single penny. i decided to check the old card that my father once gave me, but the banker turned pale and whispered: “madam… you need to see this!” i froze in shock when i found out that
