Reversing Alzheimer’s Disease by Restoring Brain Energy Metabolism: Implications of a 2025 Breakthrough Study
For decades, Alzheimer’s disease (AD) has been widely regarded as an irreversible neurodegenerative disorder. Most therapeutic strategies have therefore focused on slowing cognitive decline rather than restoring lost brain function. However, a December 2025 study published in Cell Reports Medicine directly challenges this long-standing assumption by demonstrating that even brains with advanced Alzheimer’s-like damage may retain a surprising capacity for recovery when a core metabolic deficit is corrected.
In this study, researchers examined postmortem human Alzheimer’s brain tissue alongside two well-established mouse models representing distinct pathological drivers of the disease: one dominated by amyloid-β pathology and the other by tau pathology. Despite their different genetic and molecular origins, all samples shared a striking common feature—severe disruption of nicotinamide adenine dinucleotide (NAD⁺) balance. NAD⁺ is a central molecule required for cellular energy production, mitochondrial function, DNA repair, and neuronal survival. While NAD⁺ levels normally decline with aging, the deficit observed in Alzheimer’s brains was far more profound, suggesting that impaired energy metabolism is not merely a consequence of neurodegeneration but a central driver of disease progression.
To determine whether this metabolic failure was reversible, the researchers intervened after significant pathology had already developed. Using a targeted pharmacological compound known as P7C3-A20, they restored NAD⁺ balance in the brains of mice with advanced Alzheimer’s-like disease. The outcomes were remarkable. Treated animals showed repair of synaptic function, reduction of neuroinflammation, stabilization of the blood–brain barrier, and—most notably—full recovery of learning and memory performance. These results indicate not just symptomatic improvement, but restoration of core neural processes that are typically considered permanently lost in late-stage disease.
Crucially, the study also reported normalization of blood levels of phosphorylated tau-217 (pTau-217), a biomarker currently used in human clinical practice to track Alzheimer’s pathology. The reversal of this biomarker provides biochemical evidence that disease-driving processes themselves were corrected, rather than merely bypassed. This distinction is fundamental: it suggests that restoring cellular energy balance can actively reverse pathological cascades associated with both amyloid- and tau-driven neurodegeneration.
From a conceptual standpoint, these findings represent a paradigm shift in Alzheimer’s research. Rather than treating amyloid plaques or tau tangles as isolated targets, the study positions disrupted energy metabolism as a unifying upstream mechanism that links diverse pathological features of the disease. By reframing Alzheimer’s as, at least in part, a disorder of metabolic collapse, the research opens new therapeutic avenues that extend beyond traditional protein-centric approaches.
At the same time, important limitations must be acknowledged. The intervention was tested exclusively in animal models, and the compound used has not yet been validated in human clinical trials. Mouse models, while informative, cannot fully replicate the complexity of human Alzheimer’s disease, particularly in its late stages. Therefore, these results do not yet demonstrate that advanced Alzheimer’s can be reversed in humans. Instead, they establish biological plausibility and proof of principle that such reversal may be possible under specific conditions.
Nevertheless, the implications are profound. If future clinical trials confirm that restoring NAD⁺ balance can repair neural function in humans, Alzheimer’s disease may no longer be viewed as inevitably progressive and irreversible. Instead, it could become a condition in which meaningful recovery is possible, even after significant damage has occurred.
In conclusion, the December 2025 study published in Cell Reports Medicine provides compelling preclinical evidence that advanced Alzheimer’s-like pathology can be functionally reversed by restoring brain energy metabolism (Cell Reports Medicine, 2025). By demonstrating recovery of cognition, synaptic integrity, and disease biomarkers, the research reframes Alzheimer’s disease as a potentially reversible disorder under the right metabolic conditions. While human trials are essential before clinical conclusions can be drawn, this work fundamentally shifts how the future treatment of Alzheimer’s disease may be envisioned.
News in the same category

Top Signs Your Body is Toxic and What to Do About It
How Water Fasting Can Regenerate the Immune System, Slow Aging, Reduce Heart Attack Risk and More

Colon Cleansing With Kefir and Flaxseed Meal
CRISPR-Edited Islet Cell Transplantation: A New Therapeutic Horizon for Type 1 Diabetes

Fermented Royal Jelly and Enhancement of Human Mucosal Immunity
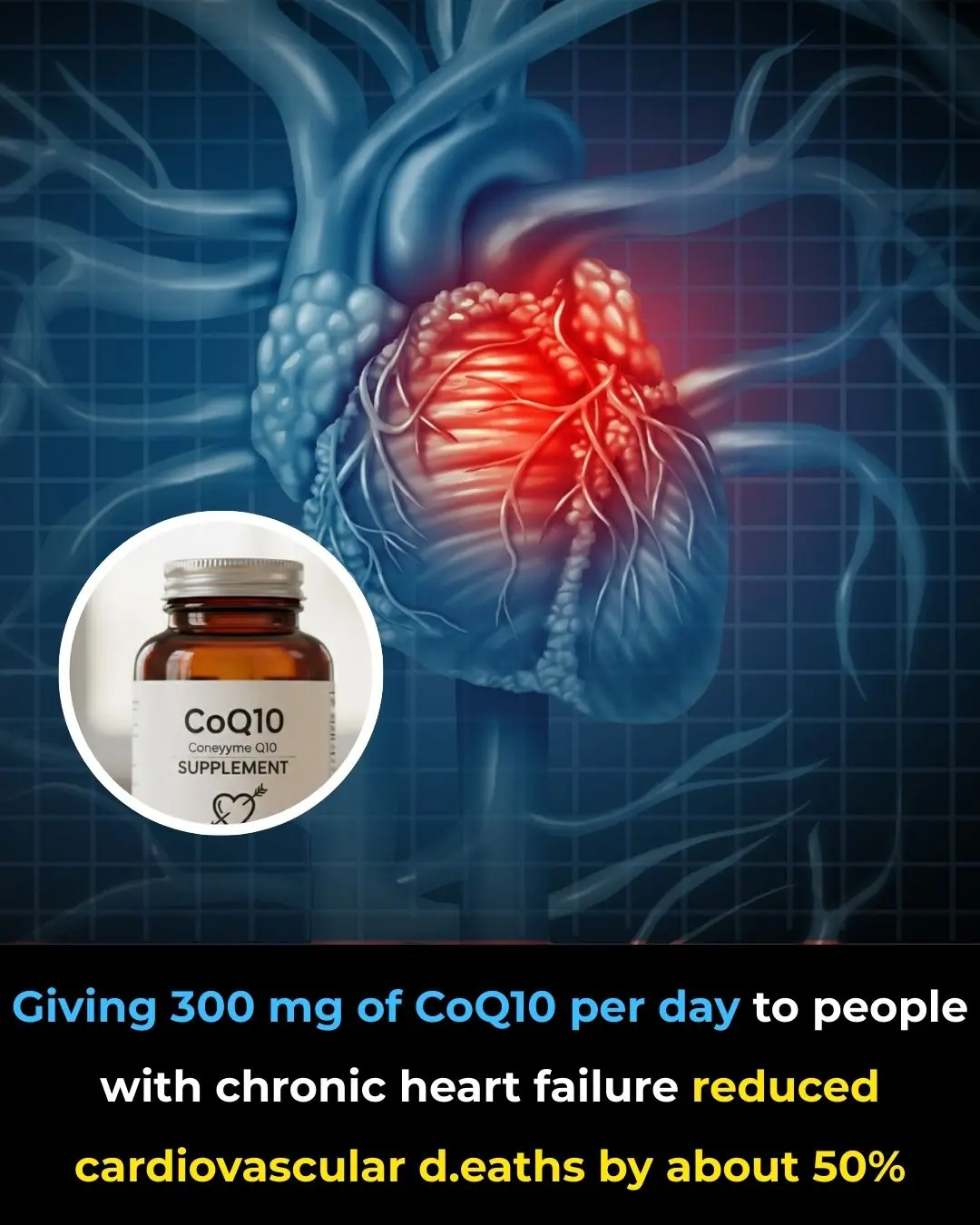
Coenzyme Q10 Supplementation and Survival in Chronic Heart Failure: Evidence from the Q-SYMBIO Trial

Vitamin C Status, Fat Oxidation, and Fatigue: Evidence from a Human Metabolic Study

Household Clutter as a Chronic Stressor for Women: Psychological and Physiological Evidence

Why Your Cough Gets Worse at Night — and What to Do About It

What Vitamin Deficiency Causes Dry Lips?

A man's liver was riddled with holes like a honeycomb due to regularly eating one type of fish: Doctors advise eating as little as possible of these four types of fish because they contain carcinogens

Seven Common Habits Linked to Cancer After the Age of 40

Five Highly Toxic Meats That Should Be Avoided

Vinegar Consumption and Reduced Risk of Calcium Oxalate Kidney Stones: Evidence from a Pilot Human Study

11 Health Warnings Your Fingernails May Be Sending

Bloated Stomach: 8 Common Reasons and How to Treat Them (Evidence Based)
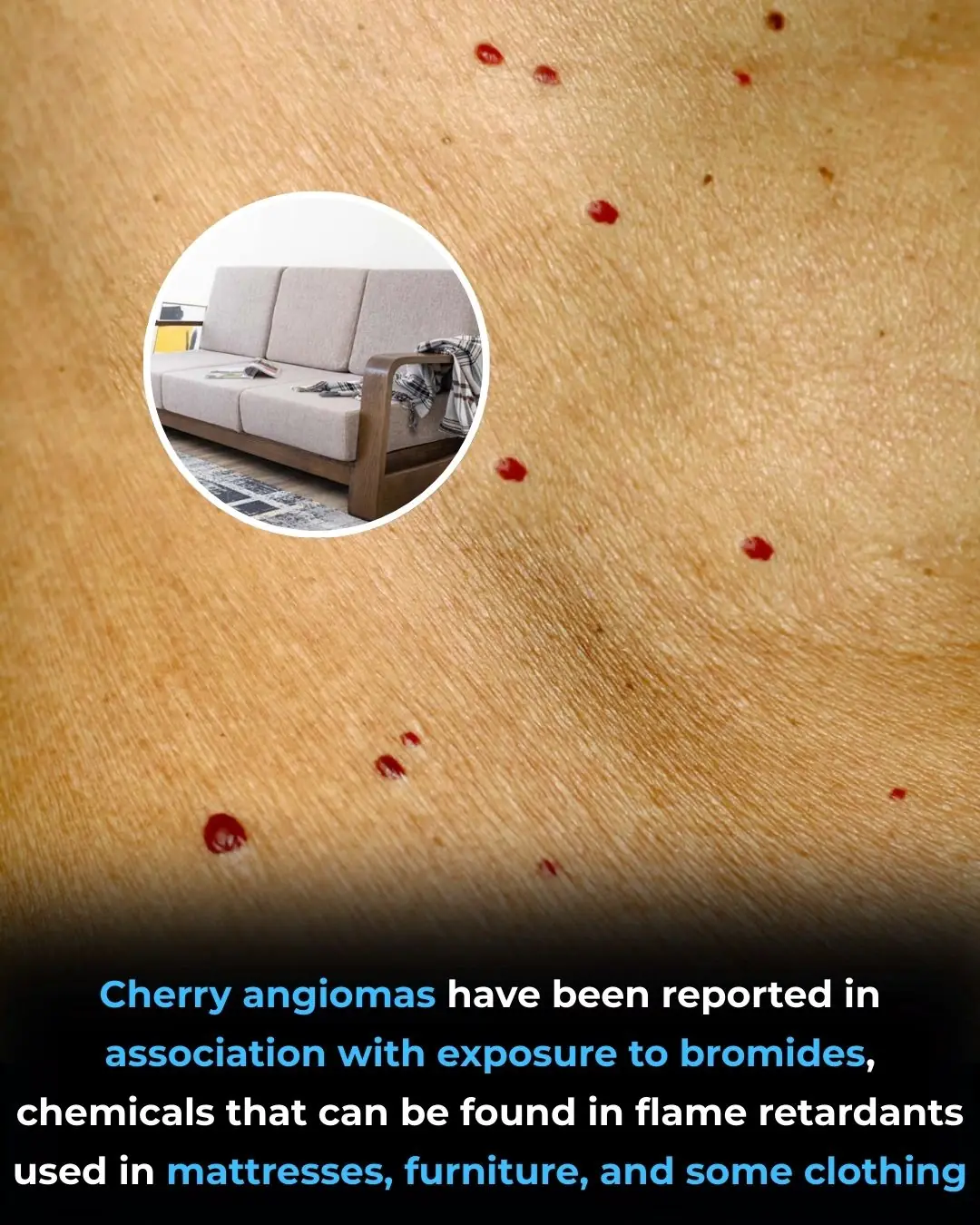
Occupational Bromide Exposure and the Development of Multiple Cherry Angiomas: Insights from a Case Report

How to Use Castor Oil to Regrow Eyelashes and Eyebrows
News Post

When you reach 60, the best way to live a healthy and long life isn't through excessive exercise, but through these four habits

My nana taught me this hack to get rid of puffy eyes in 2 mins with 0 work. Here’s how it works

Shoulder Pain from Sleeping: Causes, Solutions and More

Top Signs Your Body is Toxic and What to Do About It

How Water Fasting Can Regenerate the Immune System, Slow Aging, Reduce Heart Attack Risk and More

Ja Rule Says He's 'Very Happy and Excited' to Become a Grandfather, Adds It's a 'Blessing' (Exclusive)

Colon Cleansing With Kefir and Flaxseed Meal

David Spade says he spent 25 years trying to get Eddie Murphy to stop hating him: 'He was a hero'

Beyoncé Is Now A Billionaire

Former Fibrebond CEO Shares $240 Million in Bonuses After $1.7 Billion Sale

Texas’ top prosecutor gives his explanation after 34 dead bodies pulled from same Houston bayous sparking serial killer fears

CRISPR-Edited Islet Cell Transplantation: A New Therapeutic Horizon for Type 1 Diabetes

Fermented Royal Jelly and Enhancement of Human Mucosal Immunity

Coenzyme Q10 Supplementation and Survival in Chronic Heart Failure: Evidence from the Q-SYMBIO Trial

Vitamin C Status, Fat Oxidation, and Fatigue: Evidence from a Human Metabolic Study

Household Clutter as a Chronic Stressor for Women: Psychological and Physiological Evidence

Hercules: The Starving Puppy Who Found Love and Life Again

🐾 In Memory of Peyton
