
FDA-Approved Cancer Drug Shown to Halt Parkinson’s Disease

What if a drug originally designed to fight cancer could also slow down the relentless march of Parkinson’s disease? At first, it sounds like the plot of a medical thriller, but this idea is fast becoming a scientific reality. Parkinson’s disease, which affects more than 10 million people worldwide, has long been viewed as an incurable condition that strips away movement, independence, and identity. Current treatments provide partial relief from symptoms, yet do little to halt the deeper biological damage driving the disease.
In a surprising twist, researchers have found that a cancer drug already approved by the U.S. Food and Drug Administration (FDA) may achieve what no existing Parkinson’s therapy has managed: disrupt the disease process itself. Early findings suggest it can reduce toxic protein buildup in the brain and even protect or restore dopamine-producing neurons—the very nerve cells most affected by Parkinson’s.
What Is the Drug and How Does It Work?
The drug at the center of this breakthrough is nilotinib, originally developed to treat chronic myeloid leukemia (CML). In cancer, it works by blocking proteins that fuel malignant cell growth. But in the brain, nilotinib seems to perform an entirely different role: jumpstarting autophagy, the cell’s internal “clean-up crew.” In Parkinson’s, harmful proteins such as alpha-synuclein accumulate, clogging neurons and contributing to their death. Nilotinib enhances the cell’s ability to clear away this buildup—like unclogging a blocked waste chute that has been jammed for years.
Even more intriguing, preliminary evidence shows nilotinib may also stimulate dopamine production. Dopamine is a critical neurotransmitter responsible for movement, motivation, and mood. Its gradual loss causes many of Parkinson’s hallmark symptoms, from tremors and stiffness to cognitive decline. Researchers describe nilotinib’s dual effect—protein clearance and dopamine restoration—as “remarkably promising.” Because the drug crosses the blood–brain barrier, a major hurdle in neurological medicine, it holds an advantage over many other experimental therapies.
The Science Behind the Breakthrough

This discovery builds on years of research. Scientists knew that Parkinson’s brains were littered with toxic protein clumps, especially alpha-synuclein. In laboratory studies, nilotinib triggered autophagy in neurons, reducing these clumps and protecting dopamine-producing cells. Encouraged by such results, Georgetown University launched a small phase 1 clinical trial. Patients who received low doses of nilotinib not only showed improvements in movement, but also in mood and cognitive ability. Brain scans revealed reduced toxic proteins and possible signs of restored dopamine signaling.
Crucially, these trials used doses far below those prescribed for cancer, reducing the risk of side effects. Though early and limited in scale, the biological markers pointed strongly in the right direction.
What This Means for Patients

For people living with Parkinson’s, each day is a balancing act between tremors, stiffness, fatigue, and the emotional burden of decline. Medications such as levodopa temporarily ease symptoms but do nothing to stop progression. That’s why nilotinib’s potential is so powerful—it targets the underlying disease process. In early trials, some participants reported better movement, sharper thinking, and improved mood. For patients, such changes, though modest, can be life-changing.
If larger studies confirm these effects, nilotinib could represent the first real attempt not just to mask Parkinson’s symptoms, but to slow or halt its advance. This would buy patients more time, preserve independence, and reshape treatment strategies worldwide.
Limitations and What Comes Next
Still, caution is necessary. The existing clinical trials have been small, involving fewer than 100 participants. Long-term effectiveness and safety remain unknown. Because nilotinib was designed for leukemia, it carries potential risks—including heart and liver complications—especially at higher doses. Researchers are now testing whether lower doses can be both effective and safe for older adults with Parkinson’s, many of whom already face multiple health challenges.
Moreover, Parkinson’s is not a single disease but a spectrum of related disorders. What benefits one patient group may not work for another. Nilotinib is unlikely to be a silver bullet, but it may become a key part of a multi-drug approach. Several large, multicenter trials are already underway to measure nilotinib’s impact on motor skills, cognition, and long-term disease progression.
For now, doctors strongly advise patients not to seek nilotinib outside of clinical trials. Its use for Parkinson’s remains experimental, and only controlled studies can determine its true potential.
The Bigger Picture: Drug Repurposing in Neurology

Nilotinib’s story highlights a broader trend in medicine: drug repurposing. Developing a new drug from scratch can take over a decade and cost billions. Repurposing an existing drug shortens this timeline, since safety data already exist. For neurodegenerative diseases—where time is critical—this approach can be transformative. Beyond Parkinson’s, researchers are testing diabetes, HIV, and cholesterol drugs for possible benefits in Alzheimer’s, ALS, and Huntington’s disease.
Nilotinib is particularly exciting because it penetrates the brain and promotes autophagy, mechanisms relevant not just to Parkinson’s but to other protein-aggregation disorders as well. This opens the door to precision treatments that combine existing drugs in new, targeted ways.
A New Frontier in Parkinson’s Research
Nilotinib may have started as a cancer drug, but its potential to alter the course of Parkinson’s disease is rewriting expectations in neurology. For decades, research has focused on symptom control. Now, for the first time, scientists are daring to attack the disease at its roots.
Much work remains before nilotinib could reach everyday patients. Yet the very possibility that a drug already sitting in pharmacies might protect neurons and slow neurodegeneration is revolutionary. It reminds us that sometimes, innovation isn’t about inventing something entirely new, but about seeing familiar tools in a new light.
For patients, caregivers, and scientists alike, nilotinib represents more than hope—it is a tangible step toward a future where Parkinson’s might not just be managed, but confronted at its core. The path is still long, but the journey has begun, and for millions of people, that makes all the difference.
News in the same category


Teacher arrested after viral 'poop spray' prank costs school $55,000

Charlie Kirk's last YouTube video shows eerie final debate at university

Man who bought laundromat for $15,000 shares exactly how much money business made him in 3 days

MrBeast officially cast in major new movie set for 2027 release

The TV show host didn't hold back
New Study Warns COVID-19 And Flu Might Awaken Dormant Cancer Cells In The Lungs

$42,000,000,000 reason Sam Altman, Tim Cook and other tech bosses just met with the British Royals

Fans cancel Disney+ subscriptions after their response to Jimmy Kimmel's Charlie Kirk comments

Meta launch RayBan smart glasses with 'groundbreaking' new feature

Politicians 'condemn' Elon Musk over 'dangerous' remarks made in UK protest

FBI announces it's investigating more than 20 Discord accounts in relation to Charlie Kirk murder

Man Won $158 Million On Lottery And Collected Winnings In Scream Mask So No One Found Out

Woman who had baby using 'free sperm from Facebook' issues warning to other women

Shocking dark side of the world's least visited country that stretches just 8.1 square miles

Tyler Robinson's mom makes shock admission in official Charlie Kirk murder court documents

Father of teen who died by suicide after ChatGPT 'encouraged' him gives heartbreaking testimony

The ultimate battle of faith versus tech
Man caught 'cheering' in crowd after Charlie Kirk killing breaks silence
News Post
Game-changing 'hidden' features in iOS 26 update you've probably missed
X-rays Reveal Hundreds of Golden Threads Inside a Patient with Severe Knee Pain — Here’s the Reason Why

Blind Man Can Now See Through His Tooth After Losing Sight 20 Years Ago – He Explains How It Works

Harvard expert issues chilling warning that strange object heading towards Earth may be a ‘mothership'

Nvidia's futuristic 'robot brain' officially goes on sale to the public for insane price

These 4 places are "bacteria nests" in the rice cooker, need to be cleaned every month

🌿 Wash Your Hair with This Herb and Watch Baby Hairs Grow Back Fast — Say Goodbye to Hair Fall

🪵 Don't Wash Moldy Wooden Cutting Boards with Soap—Try This 5-Minute Natural Cleaning Hack Instead

🌾 Ginger and Rice Water Hair Treatment: A Natural Secret for Fast Growth, Thicker, Shinier Hair

Colgate Toothpaste for Face Whitening: The Secret Combo of Tomato and Colgate 🍅✨

Emotional Starbucks Employee Reaction Over Long-Hour Shift Sparks Debate

Shocking Discovery at Her Son’s Bed Leaves Mom Terrified
If You Have These Two ‘Dimples’ on Your Lower Back, This is What They Mean

How My Nana’s Baking Soda Skincare Routine Became My Favorite Beauty Secret

Popular Drink Could Be Permanently Staining Your Teeth Yellow, Experts Say

Powerful Natural Remedies for Ear Infections That You Can Try at Home
CRISPR Breakthrough: Scientists Achieve Complete HIV Eradication in Lab Cells

Northern lights alert : the best displays in years could be coming
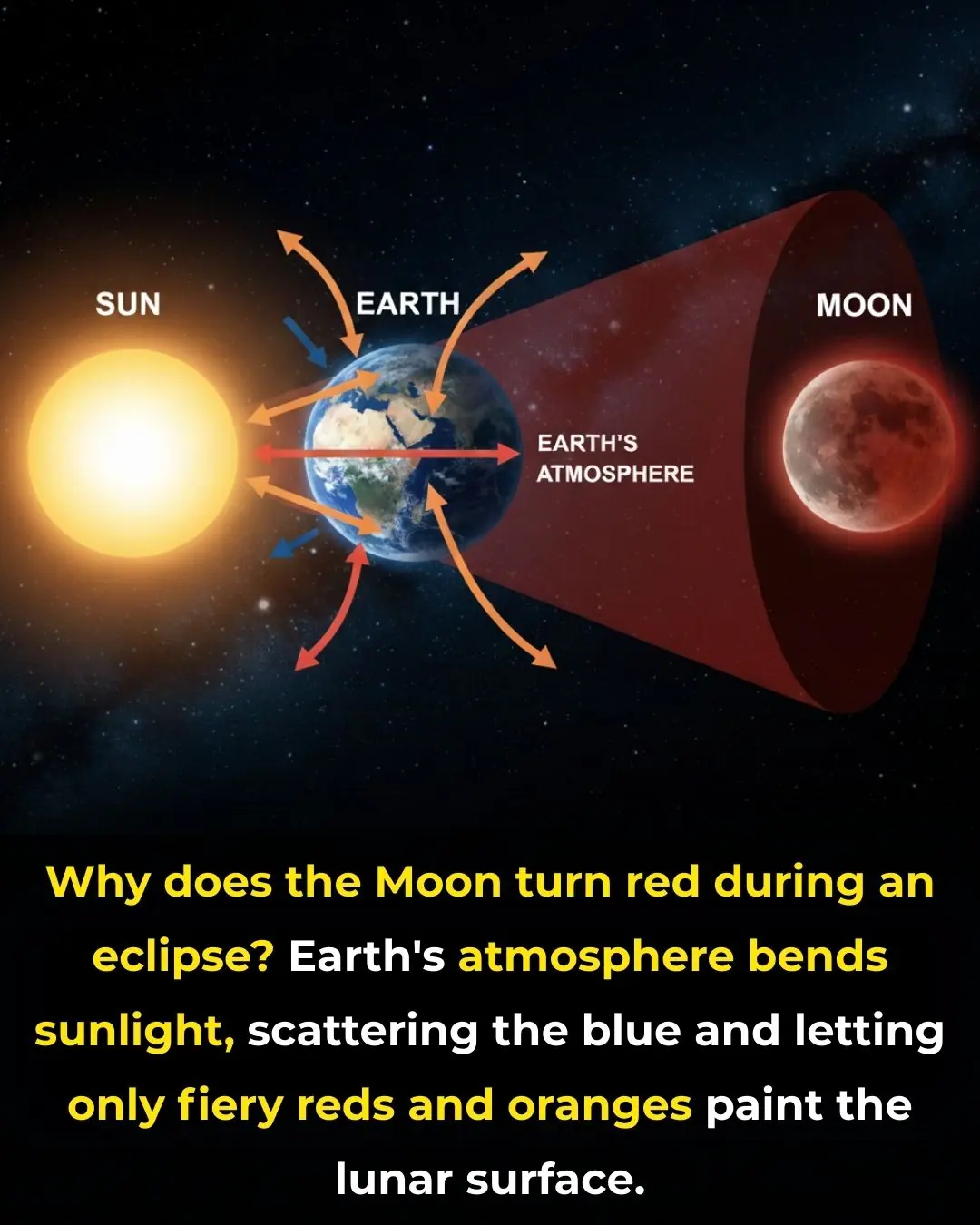
The Science Behind the Blood Moon: How Earth’s Shadow Creates the Red Glow
