Scientists Say This New Stem Cell Treatment Could End Type 1 Diabetes for Good

For decades, Type 1 diabetes has been one of those chronic conditions that modern medicine could control—but never truly cure. Those living with this autoimmune disease must meticulously track their blood sugar levels and administer insulin, often multiple times a day. It’s more than just a daily inconvenience; it’s an exhausting, lifelong commitment that shadows every meal, activity, and moment of rest.
But now, a remarkable scientific breakthrough is offering real hope to millions. A recent clinical trial has opened the door to a future where people with Type 1 diabetes might no longer be dependent on insulin injections or pumps. For the first time, scientists have found a way to potentially restore natural insulin production—not just manage symptoms, but actually reverse the disease’s core deficiency. And at the heart of this innovation? Stem cells.
Stem Cells: The Body’s Master Craftsmen
To understand why this breakthrough is so promising, it’s essential to grasp the power of stem cells. These are undifferentiated cells—biological blank slates—with the unique ability to transform into virtually any other type of cell in the body, including the insulin-producing beta cells found in the pancreas.
For years, researchers have dreamed of using stem cells to regenerate damaged organs or tissues, and now, that dream is becoming tangible. In the case of Type 1 diabetes, where the immune system destroys beta cells, the ability to grow new ones from stem cells could be nothing short of revolutionary.
The Trial That Could Change Everything
The clinical trial, spearheaded by Vertex Pharmaceuticals, involved 12 patients diagnosed with Type 1 diabetes. These individuals received an infusion of specially developed pancreatic islet cells, cultivated from pluripotent stem cells in a laboratory setting. The therapy, known by its clinical name zimislecel, represents years of groundbreaking research in regenerative medicine.
Under the leadership of Dr. Trevor Reichman, a renowned transplant surgeon at the University of Toronto, the research team sought to answer a pivotal question: could lab-grown islet cells survive and function inside the human body like natural pancreatic cells?
The answer, astonishingly, was yes.
One Year Later: Results That Defied Expectations
Fast-forward 12 months, and the outcomes stunned even the most optimistic researchers. Of the 12 participants, 10 were able to stop using insulin entirely. They no longer needed injections, pumps, or constant glucose monitoring—because their own bodies had resumed natural insulin production.
The remaining two participants also showed substantial improvement in blood sugar regulation, even though they didn’t achieve full insulin independence. This suggests the therapy may still benefit patients across a spectrum of responses, and future refinements could make it even more effective.
What’s especially encouraging is the precision of the new islet cells. They didn’t flood the bloodstream with insulin randomly. Instead, they responded intelligently—producing just the right amount of insulin in response to blood sugar fluctuations. This level of accuracy is crucial, given the risks of both hypoglycemia and hyperglycemia.
How Zimislecel Therapy Works
The zimislecel treatment involves the transplantation of insulin-producing islet cells into the patient’s liver, where they settle and begin functioning like a natural pancreas. Unlike donor transplants, which are limited in availability and often involve long waitlists, these lab-grown cells can be produced on demand and customized for each patient’s needs.
This is a monumental shift. It takes regenerative therapy out of the realm of experimental ideas and into something scalable and potentially global.
However, there’s an important caveat.
The Cost of Protection: Immunosuppressive Therapy
Because the body’s immune system naturally attacks foreign cells—including helpful ones—patients must take immunosuppressive drugs to prevent rejection of the transplanted islet cells.
These medications come with their own set of challenges: they can impair kidney function, increase susceptibility to infections, and compromise the immune response to other diseases. While the stem cell treatment itself has not caused any serious side effects so far, the reliance on immune suppression creates a new layer of medical complexity.
Researchers are now exploring ways to engineer islet cells that could evade immune detection, potentially eliminating the need for immunosuppressants altogether. If they succeed, it could be the final key to a true cure.
A Sobering Reminder: Lives Lost Along the Way
Not all stories from the trial ended in triumph. Two participants died during the course of the study. While their deaths were ruled unrelated to the stem cell therapy, the loss is a poignant reminder of the risks and realities of clinical research. Medical progress is rarely a straight path—it’s filled with setbacks, sacrifices, and sobering truths.
What Comes Next: Phase 3 Trials and Beyond
Encouraged by the success of the initial trial, Vertex is moving forward with Phase 3 clinical trials, the final major hurdle before FDA approval. This stage will involve a much larger group of participants and even more rigorous testing to confirm the treatment’s effectiveness and long-term safety.
If it passes, this therapy could become a standard treatment for Type 1 diabetes, potentially available to millions. Because the therapy is not constrained by human donor supply, it could offer equitable, scalable access to patients around the world—something that insulin therapy has never quite achieved, particularly in low-income regions.
A Global Shift in the Fight Against Diabetes
Currently, about 8.4 million people globally are living with Type 1 diabetes. In many countries, access to insulin is limited, unaffordable, or inconsistent—leading to countless preventable complications and deaths. A stem cell therapy that replaces the need for insulin could have an immeasurable impact on public health.
Beyond improving quality of life, this approach could significantly reduce the risk of long-term complications like nerve damage, blindness, kidney failure, and cardiovascular disease—conditions that not only harm individuals but place a heavy financial burden on healthcare systems.
Final Thoughts: From Management to Reversal
Every now and then, science reaches a turning point—one of those rare moments where hope meets reality. Zimislecel might represent exactly that: the shift from managing Type 1 diabetes to actually reversing it.
While calling it a full “cure” may still be premature—especially with the need for immunosuppression and further long-term data—the therapy undeniably brings us closer than ever to that goal.
The next few years will be crucial. But for now, the image of 10 people living without insulin for the first time in years paints a powerful picture: not just of what science can do, but of what’s possible when medicine dares to dream bigger.
This isn’t the end of the story—but it’s the most hopeful chapter yet.
News in the same category

What Your Feet Are Telling You
6 Health Benefits of Sleeping In a Cold Room and How to Make it Cooler- And Why You May Not Want to Use a Fan
10 Symptoms of Diabetes That May Show Up In Your Feet
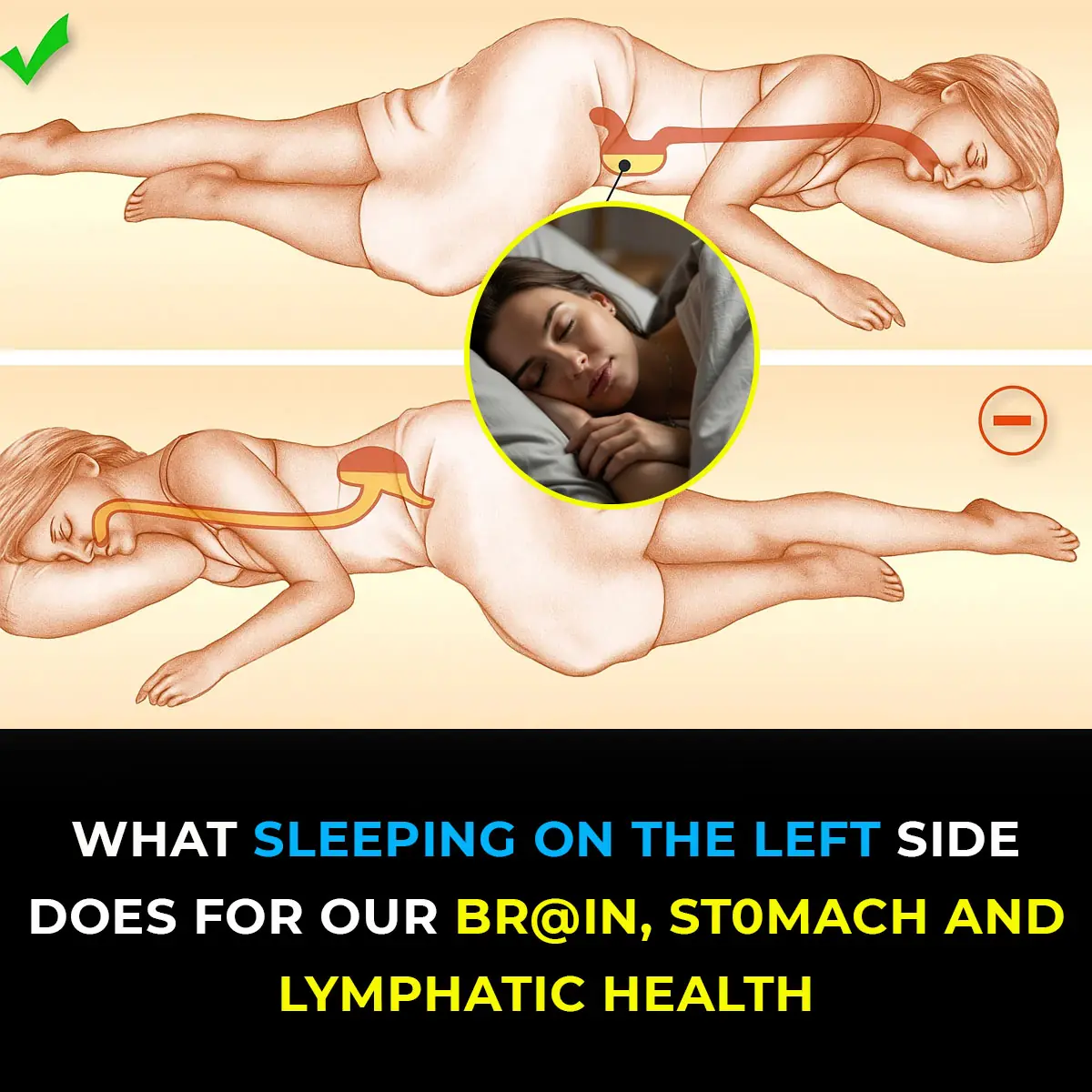
This is what sleeping on the left side does for our brain, stomach & glymphatic health

12 Best Foods To Support Digestive and Gut Health

Study suggests anal cancer is on the rise and reveals who’s most at risk

Wife Complains of a Headache, Sleeps, and Dies Without Husband Knowing: This Type of Headache Requires Immediate Hospitalization!

Shocking Effects of Sleeping Less Than 7 Hours — What Really Happens to Your Body
Getting less than seven hours of sleep might feel harmless, but science shows it can quietly damage your body in ways you don’t expect. From hormonal imbalances to skin problems and even digestive issues, sleep deprivation affects far more than just you

Doctors Explain Why You Should Never Hold Back a Fart
On average, every person passes gas 14 to 23 times per day—it’s a natural part of being human and actually shows that your digestive system is functioning properly.
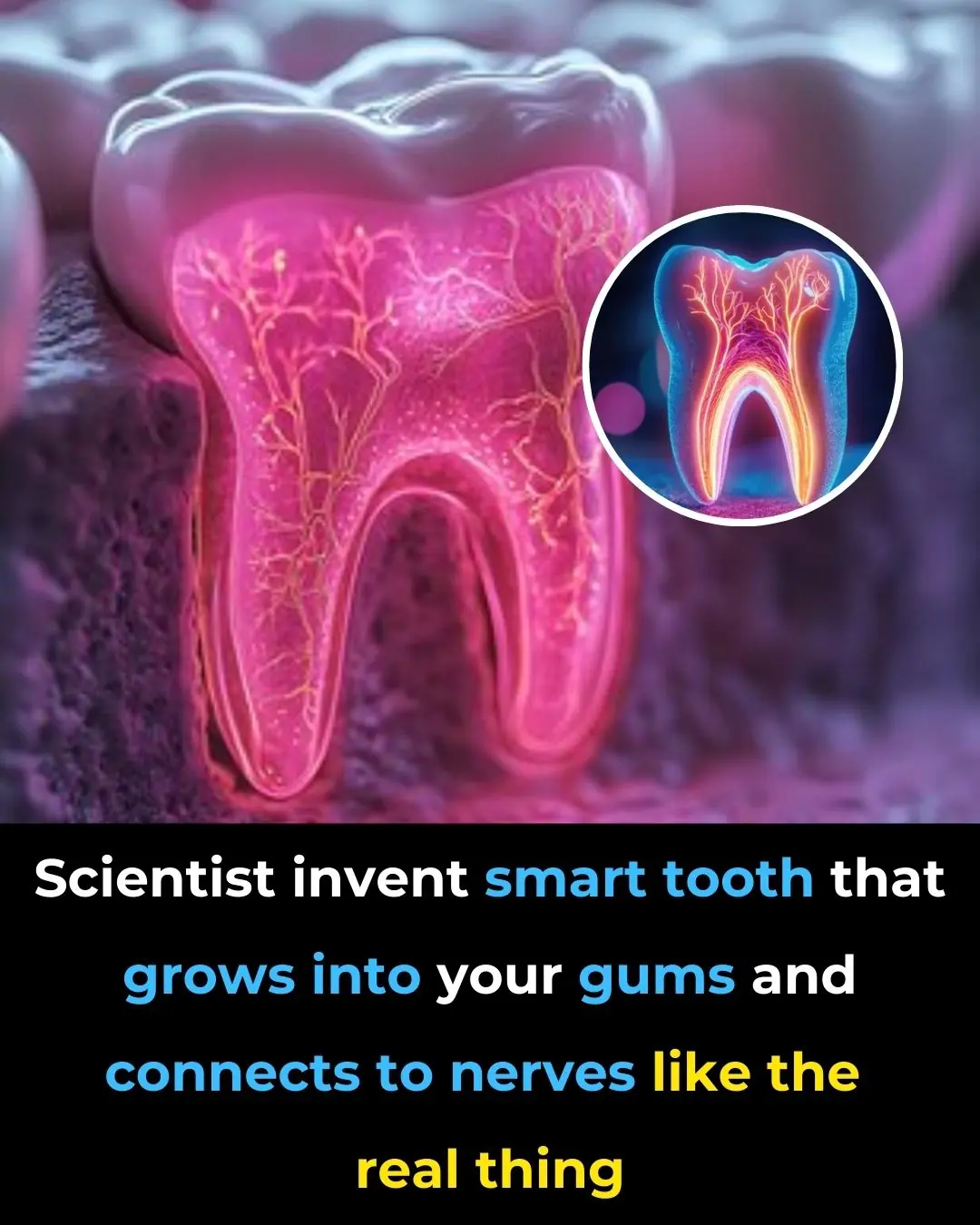
Scientists Invent Smart Tooth That Grows Into Your Gums And Connects To Nerves Like the Real Thing
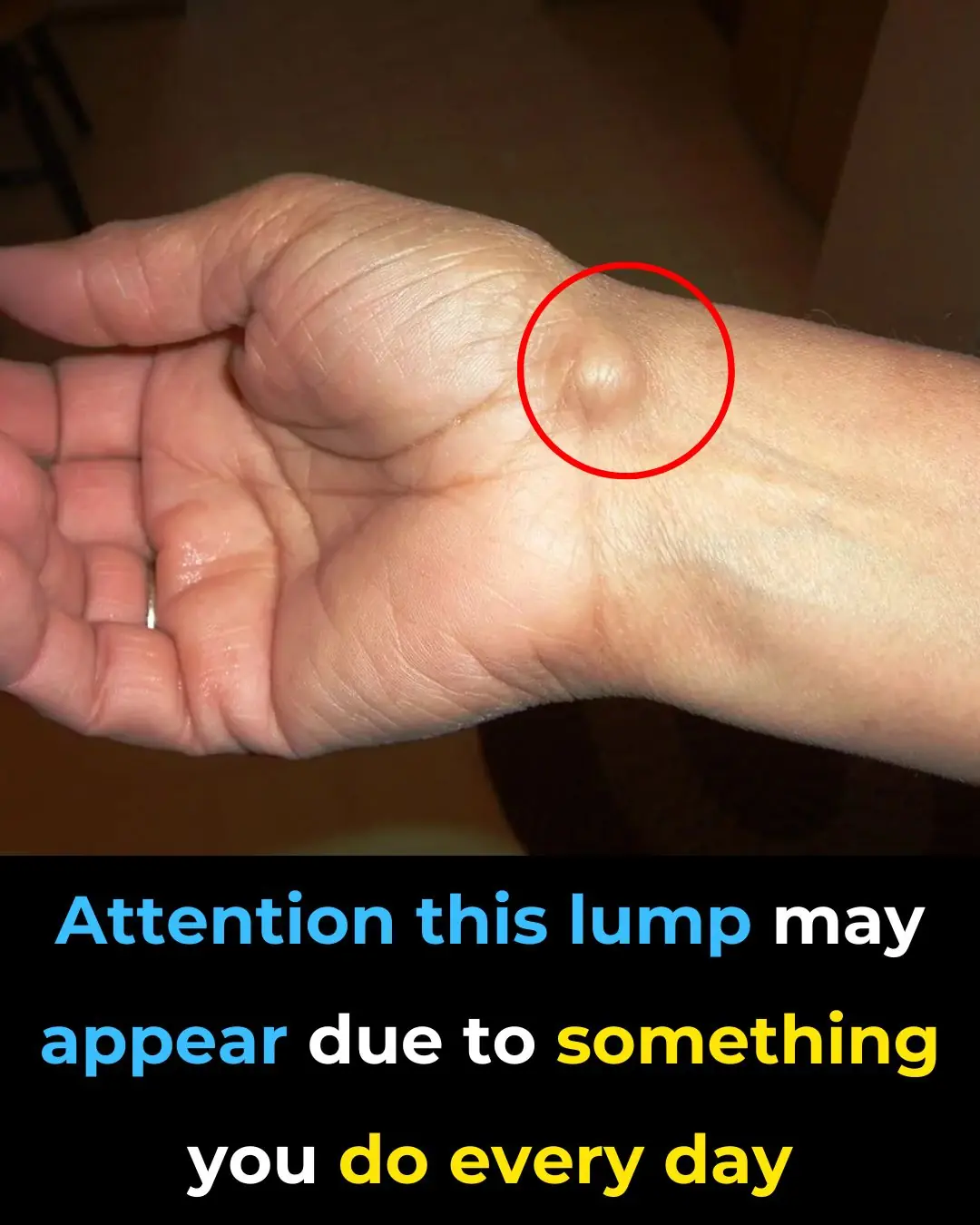
Everyday Habits That Can Cause a This Issue To Your Hands

My Nana’s Homemade Cure for Stubborn Throat Mucus That Works Every Time

Doctors Are Shocked by What Happens When You Eat Chia Seeds First Thing in the Morning
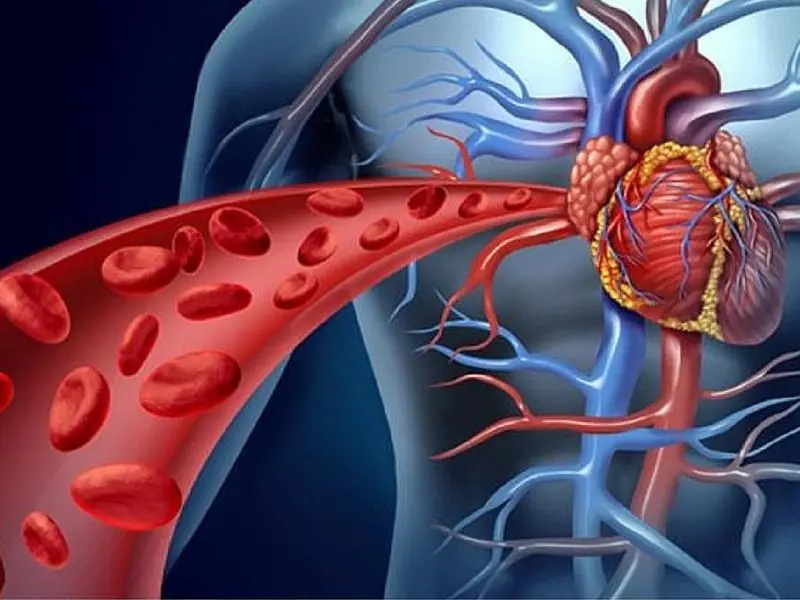
Warning Signs of Poor Blood Circulation That Are Easy to Ignore

Notice Incredible Results For Your Digestive Problems

Medical Myths Debunked: Chocolate Isn’t Heart-Healthy

Raising the Bar on Breast Cancer Screening and Management
Top 5 vitamins to supercharge circulation in your legs & feet
News Post

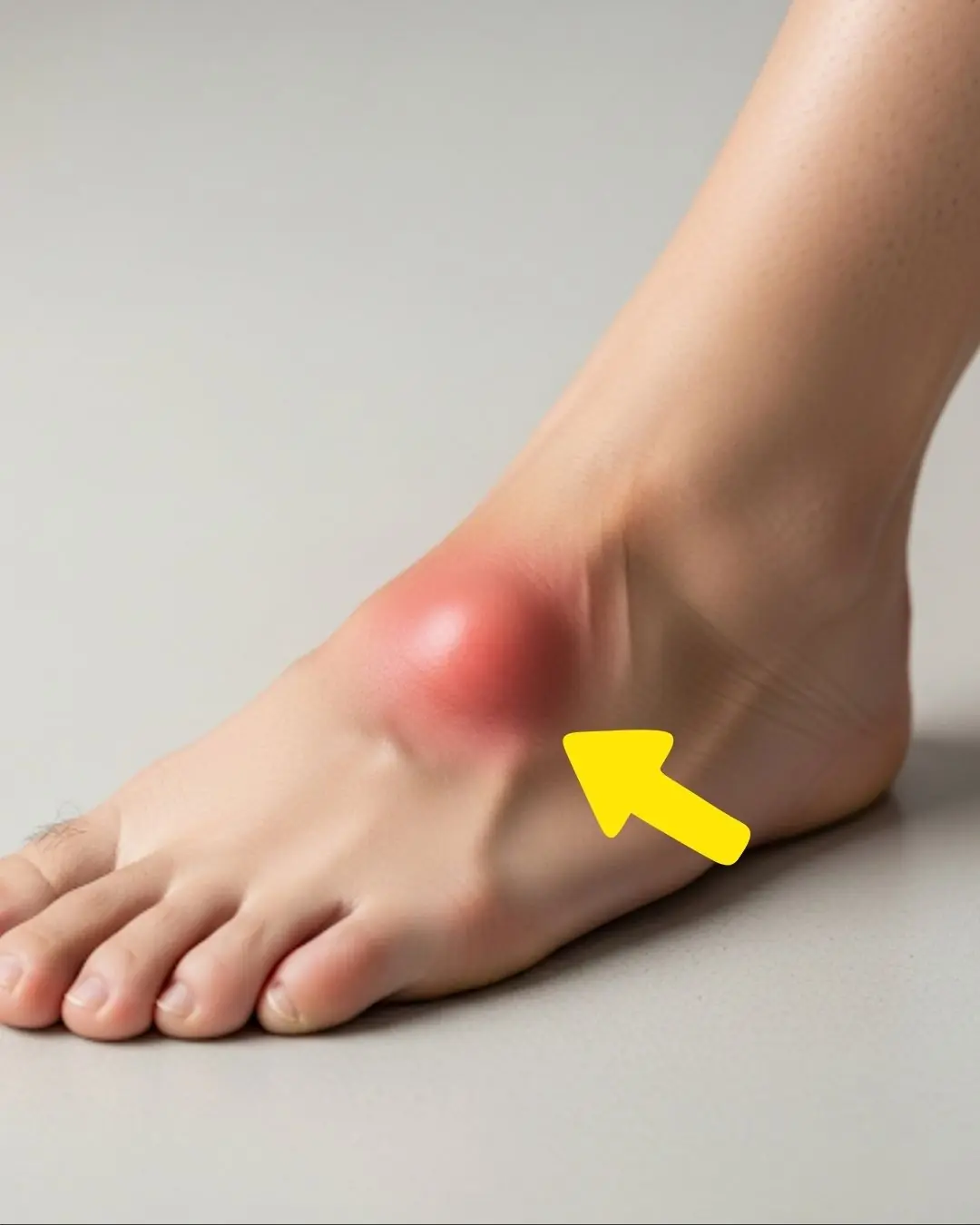
If Your Feet Swell It Is a Clear Sign

What Your Feet Are Telling You

6 Health Benefits of Sleeping In a Cold Room and How to Make it Cooler- And Why You May Not Want to Use a Fan

The Hidden Meaning Behind Leg-crossing — It’s More Than Just Comfort

10 Symptoms of Diabetes That May Show Up In Your Feet

How To Properly Dispose of Ticks

This is what sleeping on the left side does for our brain, stomach & glymphatic health

12 Best Foods To Support Digestive and Gut Health

Study suggests anal cancer is on the rise and reveals who’s most at risk

Wife Complains of a Headache, Sleeps, and Dies Without Husband Knowing: This Type of Headache Requires Immediate Hospitalization!

Why Do You Keep Waking Up Between 3 and 5 A.M.? Causes, Explanations, and What It Means for Your Health

Deadly Secrets of Ticks: How to Remove and Dispose of Them Safely Before They Harm You
Ticks may be small, but their impact on human and pet health can be enormous.

Frankenstein Rabbits With Tentacle-Like Horns Spark Invasion As Rare Virus Causes Monstrous Mutations
Although they may look frightening or pitiful, they remain a natural example of how viruses can drastically alter an animal’s appearance.

Only the Sharpest Eyes Can Find All 6 Hidden Words in This Living Room Challenge
If you love solving puzzles and challenges, this viral hidden words image is just for you!

Shocking Effects of Sleeping Less Than 7 Hours — What Really Happens to Your Body
Getting less than seven hours of sleep might feel harmless, but science shows it can quietly damage your body in ways you don’t expect. From hormonal imbalances to skin problems and even digestive issues, sleep deprivation affects far more than just you

The Hidden Purpose of That Tiny Hole in a Safety Pin Will Surprise You
That tiny hole in a safety pin isn’t just decoration - it’s proof that even the simplest everyday tools can hide smart design secrets.

Doctors Explain Why You Should Never Hold Back a Fart
On average, every person passes gas 14 to 23 times per day—it’s a natural part of being human and actually shows that your digestive system is functioning properly.

This Appears to Be a Void in Space. In Truth, It’s Full of Stars in the Making

Potentially hostile’ alien threat could attack Earth in a few months, scientists claim
