CDC's Historic Decision to End Monkey Testing: A Shift Towards More Humane and Advanced Research Models
The CDC's Historic Decision to End Monkey Testing in Laboratories: A Step Toward More Humane and Advanced Research Models
In a landmark decision announced in late 2025, the U.S. Centers for Disease Control and Prevention (CDC) has officially decided to halt all experiments involving monkeys in its laboratories. This move, which marks a significant policy shift, is viewed as a historic step away from the use of non-human primates in federal biomedical research. It is a pivotal moment for animal rights advocates, scientists, and the broader public, who have long debated the ethics of using primates in research.
The directive issued by the CDC mandates that all research involving monkeys must be phased out by the end of the year. This decision affects approximately 200 macaques housed at the CDC’s facilities in Atlanta. These macaques were previously used for a variety of research purposes, including studies on infectious diseases and vaccine development. The CDC has not provided full details on the fate of these monkeys, but there is growing public pressure for their transfer to animal sanctuaries rather than euthanization. Animal welfare organizations, including PETA (People for the Ethical Treatment of Animals), have advocated for the humane treatment of the monkeys, pushing for a life outside the laboratory environment.
The CDC’s decision is seen as a significant victory by animal rights groups and comes after years of relentless campaigning against the use of primates in scientific experiments. Many of these experiments involved high-security labs and studied infectious diseases such as HIV and hepatitis. These studies were often criticized for subjecting animals to distressing conditions, raising ethical concerns about their necessity and the scientific validity of the results.
This move by the CDC is part of a broader trend within the scientific community to reduce reliance on animal testing, especially involving primates. Growing concerns about animal suffering and the increasing availability of alternative research methods have played a key role in this shift. Advancements in technology, such as human cell cultures, organ-on-a-chip technology, and artificial intelligence (AI) modeling, are now providing more ethical and potentially more accurate methods for conducting biomedical research.
Human cell cultures, for example, offer the advantage of mimicking human physiological responses without the need for animal testing. Organ-on-a-chip technology allows researchers to simulate the behavior of human organs, providing a more accurate representation of how drugs and diseases affect humans. Moreover, AI modeling has become an invaluable tool in predicting how drugs interact with human biology, further reducing the need for animal subjects in clinical trials. These alternatives are not only more humane but are also increasingly proving to be more efficient and effective in producing reliable scientific data.
Experts in the field of biomedical research view the CDC’s decision as a potential catalyst for other government agencies, such as the National Institutes of Health (NIH) and the Food and Drug Administration (FDA), to reconsider their own use of primates in research. Although no official announcements have been made by these organizations, the CDC’s policy shift could encourage similar action in other federal research programs. As public and scientific opinion continues to evolve, it is likely that more institutions will follow suit, adopting alternatives to animal testing.
In addition to its ethical implications, the CDC’s decision reflects a broader shift in scientific practices. The decision is seen as a forward-thinking move that prioritizes humane research methods while also embracing cutting-edge technologies that promise to enhance the accuracy and reliability of scientific results. While the path forward will likely require significant investment in new research methodologies and technologies, the transition to more ethical and effective models is one that many in the scientific community and beyond welcome.
Ultimately, the CDC’s historic decision is not only a compassionate step forward for animal welfare but also a sign of evolving scientific practices that prioritize both human and animal well-being. As we move further into the 21st century, it is clear that scientific research is entering a new era—one where compassion, innovation, and ethics work hand in hand to drive progress.
Sources:
-
"CDC to End All Monkey Research Program," Scientific American, 2025. scientificamerican.com
-
"PETA Celebrates CDC Decision to End Primate Research," PETA, 2025. peta.org
-
"Organ-on-a-Chip Technology: The Future of Biomedical Research," National Institutes of Health (NIH), 2024. nih.gov
-
"AI in Drug Development: Advancements and Opportunities," Fierce Biotech, 2025. fiercebiotech.com
News in the same category


A Heartwarming Tale of Workplace Compassion: A Father's 262 Days of Paid Leave

Debunking the Myth: Why Humans Did Not Evolve from Monkeys
The Hidden Climb of Thyroid Cancer in Younger Women

56 Percent Of Americans Don’t Think We Should Teach Arabic Numerals In School

People Shocked After Finally Realizing What McDonald's Sweet 'N' Sour Sauce Is Really Made From

Eating Kimchi For 12 Weeks Helped People's Immune Cells Get Better At Spotting Viruses While Also Stopping Overreactions

Drunk Raccoon Turns Liquor Store into His Personal Bar Before Passing Out in the Bathroom

Turning Chicken Manure into Renewable Energy: The Netherlands' Circular Economy Solution
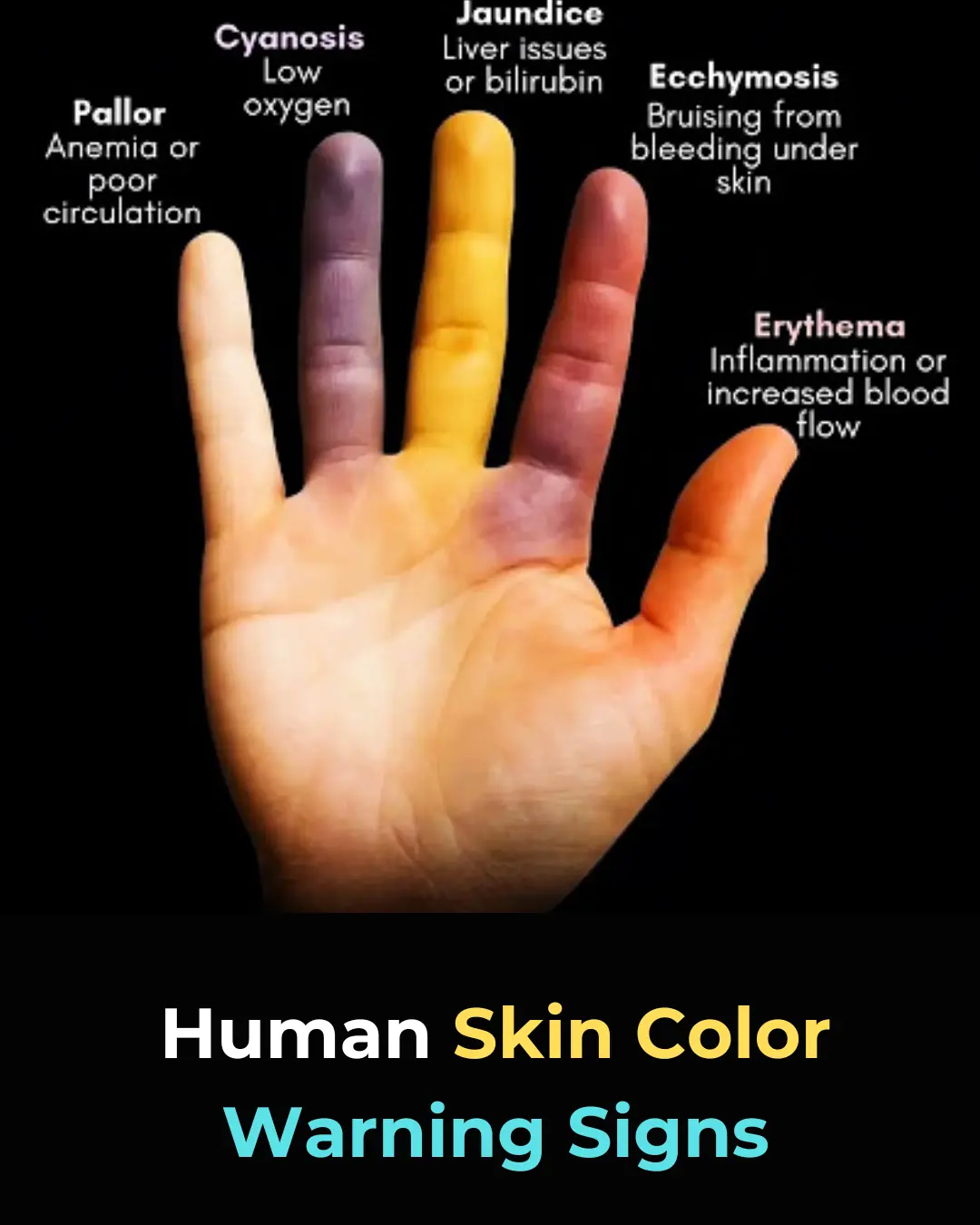
Understanding Skin Color Changes as Early Warning Signs of Health Issues

Rogfast Tunnel: Norway's Record-Breaking Undersea Highway Project

Expanding Human Perception: Exploring the Limits of Vision and Hearing Through Technology

Teen Inventor Creates Battery-Free Flashlight Powered Only by Human Body Heat

The Boy Who Walked Through Ice: How Wang Fuman Inspired the World

From –4°F to Spring in Minutes: The Incredible 1943 Spearfish Temperature Shock

Saman Gunan: The Diver Who Gave His Life to Save the Wild Boars Team

From Circus to Sanctuary: Charley the Elephant Finds Freedom After Four Decades

10 Heartbreaking Reasons Children Stop Visiting Parents

8 Mind-Bending Optical Illusions That Test Your Level of Self-Awareness
News Post

Sip Your Way to Vibrance: The Ultimate Lipton, Cloves, and Ginger Tea for Women’s Wellness
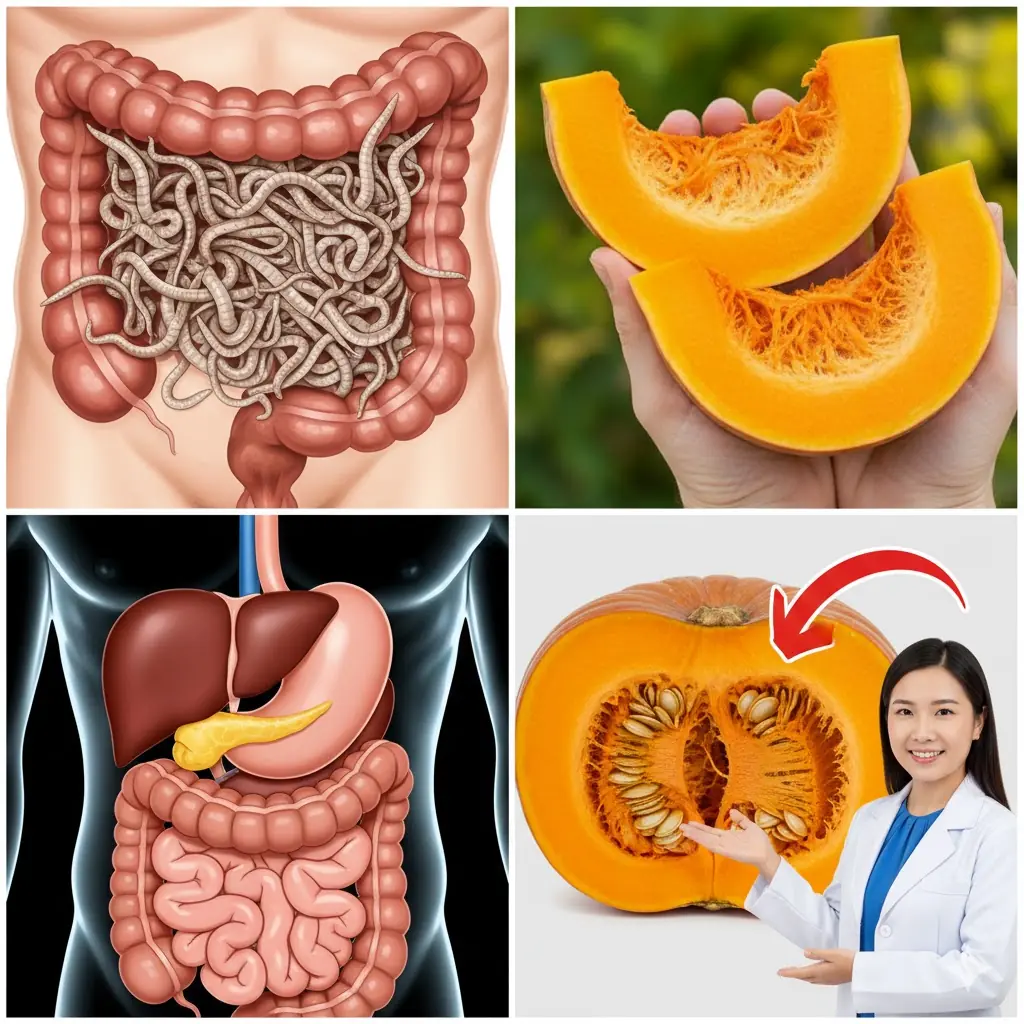
Pumpkin Seeds: Nature’s Fierce Parasite Fighters for a Healthier Gut

Tamarind: A Promising Natural Solution to Help the Body Clear Microplastics

A Heartwarming Tale of Workplace Compassion: A Father's 262 Days of Paid Leave

Debunking the Myth: Why Humans Did Not Evolve from Monkeys

The Hidden Climb of Thyroid Cancer in Younger Women

56 Percent Of Americans Don’t Think We Should Teach Arabic Numerals In School

From Rain to Runway: How Singapore’s Changi Airport Saves Over 8 Million Gallons of Water a Year

People Shocked After Finally Realizing What McDonald's Sweet 'N' Sour Sauce Is Really Made From

Eating Kimchi For 12 Weeks Helped People's Immune Cells Get Better At Spotting Viruses While Also Stopping Overreactions

Drunk Raccoon Turns Liquor Store into His Personal Bar Before Passing Out in the Bathroom

Turning Chicken Manure into Renewable Energy: The Netherlands' Circular Economy Solution
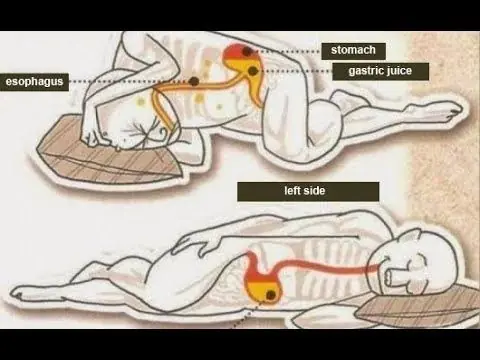
Why Sleeping on Your Left Side Is the Best Thing You’re Not Doing

Rising Tide of Change: The World’s Coastlines Are Entering a New Era

The Girl Who Said No — And Changed a Nation Forever

Understanding Skin Color Changes as Early Warning Signs of Health Issues

Rogfast Tunnel: Norway's Record-Breaking Undersea Highway Project

Expanding Human Perception: Exploring the Limits of Vision and Hearing Through Technology
