GIP Reduces Postprandial Glucose Peaks but Does Not Prevent Hypoglycaemia in Men With Type 1 Diabetes

Glucose-dependent insulinotropic polypeptide (GIP) is one of the key incretin hormones involved in glucose metabolism. Alongside glucagon-like peptide-1 (GLP-1), GIP plays a role in stimulating insulin secretion in response to nutrient intake. While incretin-based therapies have transformed the management of type 2 diabetes, their role in type 1 diabetes (T1D) remains less clear. Recent research has explored whether GIP could help mitigate postprandial glucose excursions or reduce the risk of hypoglycaemia in people with T1D, particularly in challenging real-world scenarios such as excess insulin dosing combined with physical activity.
Study Overview
In a controlled clinical study involving men with type 1 diabetes, researchers investigated the metabolic effects of an intravenous infusion of GIP under conditions designed to provoke hypoglycaemia. Participants received prandial insulin in excess of their physiological needs, followed by postmeal physical activity—a combination known to substantially increase the risk of low blood glucose levels.
To better understand GIP’s role, outcomes during GIP infusion were compared with those observed during infusion of a GIP receptor antagonist, GIP(3-30)NH₂. This antagonist blocks GIP receptor signaling and therefore serves as a useful comparator to isolate the hormone’s effects.
Effects on Hypoglycaemia
The primary finding of the study was that intravenous GIP infusion did not prevent hypoglycaemia in this high-risk setting. When prandial insulin doses were excessive and participants engaged in physical activity after eating, hypoglycaemic episodes still occurred despite the presence of GIP.
This result suggests that, in people with T1D, GIP alone is insufficient to counteract the powerful glucose-lowering effects of exogenous insulin combined with exercise. Unlike individuals with preserved endogenous insulin secretion, people with T1D lack the intrinsic beta-cell regulatory mechanisms that might otherwise allow incretin hormones to modulate insulin output in response to falling glucose levels.
Impact on Postprandial Glucose Peaks
Although GIP did not offer protection against hypoglycaemia, it did demonstrate a modest but measurable benefit in reducing postprandial glucose peaks. Compared with infusion of the GIP receptor antagonist, GIP infusion resulted in lower peak glucose concentrations after meals.
This finding indicates that GIP retains some glucose-lowering capacity in T1D, likely through mechanisms such as delayed gastric emptying, modulation of glucagon secretion, or enhanced peripheral glucose handling. However, the magnitude of this effect was limited and did not translate into improved safety in situations involving excessive insulin exposure.
Clinical Implications
The results highlight the complexity of glucose regulation in type 1 diabetes and underscore the limitations of incretin-based strategies in this population. While GIP may help smooth postprandial glucose excursions to a small extent, it does not appear to provide meaningful protection against hypoglycaemia during periods of insulin excess and physical exertion.
From a clinical perspective, these findings suggest that GIP-based therapies alone are unlikely to reduce hypoglycaemia risk in T1D. Careful insulin dose adjustment, continuous glucose monitoring, and individualized exercise planning remain the cornerstone strategies for preventing low blood glucose events.
Future Directions
Further research is needed to determine whether GIP could play a supportive role when combined with other hormonal or technological interventions, such as dual incretin agonists, closed-loop insulin delivery systems, or glucagon co-administration. Understanding how incretin hormones interact with exercise and exogenous insulin may help refine future therapies aimed at improving postprandial control without increasing hypoglycaemia risk.
Conclusion
In men with type 1 diabetes, intravenous GIP infusion modestly reduces postmeal glucose peaks but does not prevent hypoglycaemia when prandial insulin is administered in excess and followed by physical activity. These findings reinforce the challenges of achieving stable glycaemic control in T1D and suggest that, while GIP has limited metabolic benefits, it cannot replace careful insulin management in high-risk scenarios.
News in the same category

Can Anti-Inflammatory Topical Therapy Fill the Treatment Gap in Mild Hidradenitis Suppurativa?

How Swimming Rewires the Brain and Strengthens Cognitive Health

How the Right Foods Boost Immunity and Speed Up Recovery

The Science of Touch: How Hugging Improves Stress, Mood, and Immunity

How Gut Bacteria Influence Anxiety, Stress, and Emotional Well-Being
Your Brain on Walking: Why Movement Sparks Mental Clarity
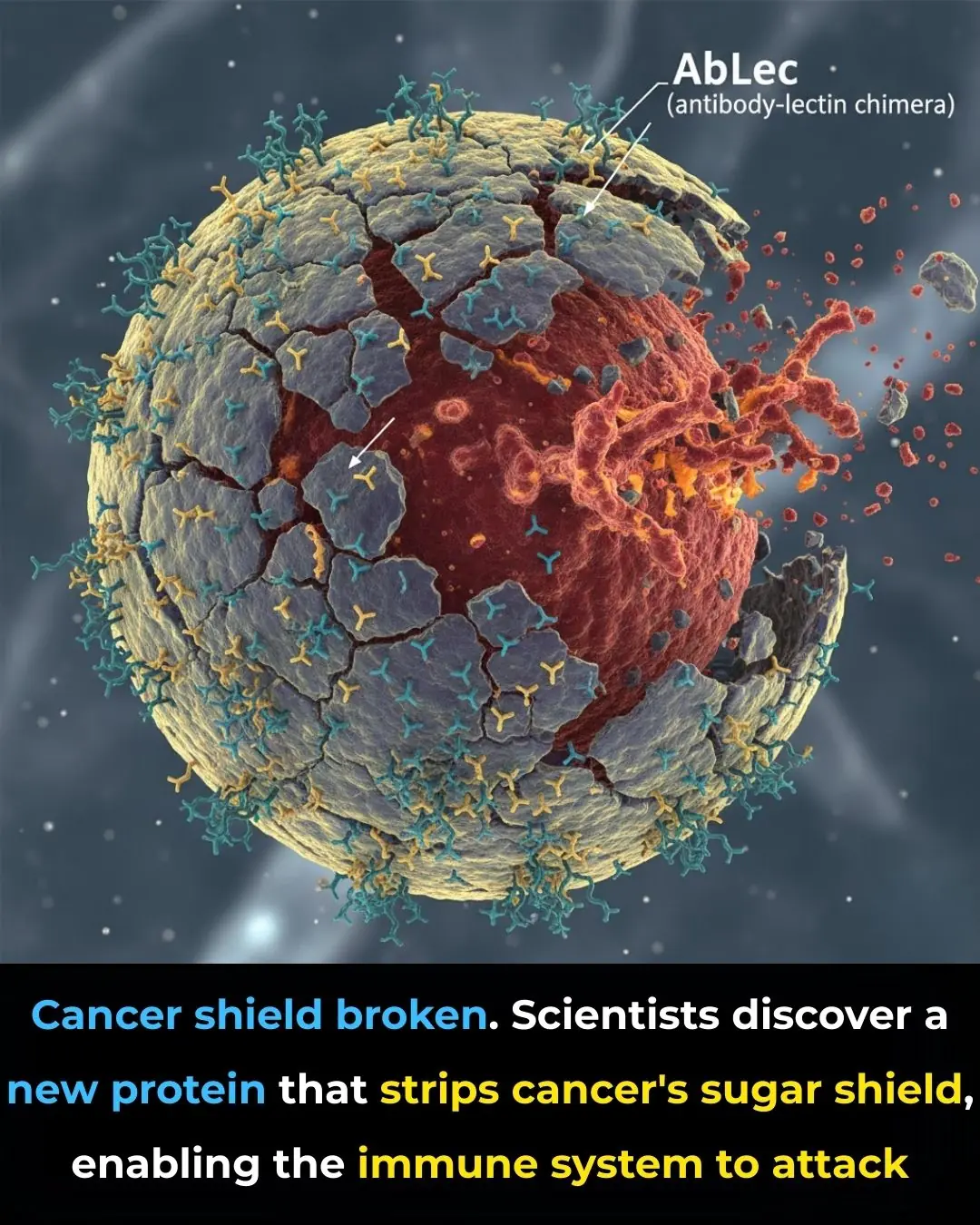
New Protein Breakthrough Helps Immune System Unmask and Destroy Cancer Cells

Using Star Apple Leaves to Treat Acid Reflux: A Traditional Remedy

Early-stage fatty liver disease: 5 obvious signs that can be noticeable on your face; ignoring them could lead to serious health consequences

Walking Barefoot at Home
Stroke and Cerebral Infarction Prevention:
If You Notice This Sign on Your Ear, Here’s What It Could Mean

5 Foods That Boost Immunity Better Than Garlic
What Is Stomach Cancer?

How the “3-2-1” Rule Can Help You

What Is the Adam’s Apple
Proven Inflammatory Foods to Avoid According to Science
14 Signs Your Blood Sugar Is Way Too High (And 14 Ways to Reduce It)
News Post

10 Scientifically Backed Reasons Why You Should Consume Ginger Everyday

Can Anti-Inflammatory Topical Therapy Fill the Treatment Gap in Mild Hidradenitis Suppurativa?

He Thought I Was Doing Nothing at Home—Until I Left for a Week and Showed Him the Truth

Feed Your Eyes: The Power of Nutrition for Better Vision

More Than Nutrition: Vegetables That Protect Your Cells

This Aloe Vera Recipe May Naturally Support Your Body’s Defense Against Bacteria and Fungi

Pharmacies Will Never Reveal This Secret – The Leaf That May Support Cancer Cell Defense | Exploring Soursop’s Natural Potential

7 Key things about Crabgrass

Guava Leaves: 12 Benefits and Uses

India Creates History at the Asian Para Youth Games 2025 🏅🇮🇳✨

Toxic Teaser Storm: Yash Redefines Cinematic Hype 🎬🔥

India’s Next Rail Leap: Vande Bharat Sleeper Train Aces Final High‑Speed Trial 🚆🇮🇳

Dashavatar: Marathi Cinema’s Historic Leap to the Academy Awards 🏆🌍✨

Supreme Court of India Reaffirms Merit in Public Employment ⚖️🇮🇳

The iBomma Shockwave: How Immadi Ravi’s Arrest Exposed a Cybercrime Empire 🎬🔥

Steaming crab often results in the claws falling off and a fishy smell, but a chef reveals: Just follow these steps and the crab will be sweet, the meat won't dry out, and it will be incredibly fragrant.

Don't put shrimp in the refrigerator right away after buying them; add one spoonful of this sweet seasoning and they'll stay fresh for a long time.

When simmering bones, you absolutely must add these three spices; the broth will be clearer and incredibly fragrant.
