Montmorency Tart Cherry Juice as an Adjunctive Therapy in Ulcerative Colitis
Montmorency Tart Cherry Juice as an Adjunctive Therapy in Ulcerative Colitis
Ulcerative colitis (UC) is a chronic inflammatory bowel disease characterized by relapsing inflammation of the colonic mucosa, leading to symptoms such as abdominal pain, diarrhea, rectal bleeding, and reduced quality of life. Although pharmacological treatments—including aminosalicylates, corticosteroids, immunomodulators, and biologics—are effective for many patients, complete disease control is often difficult to achieve, and long-term medication use can be associated with significant side effects. Consequently, there is growing scientific interest in dietary interventions that may safely complement standard medical therapy by targeting intestinal inflammation through natural bioactive compounds.
In this context, a 2025 randomized, placebo-controlled human trial conducted by researchers from the University of Hertfordshire and the University of Central Lancashire provides compelling evidence for the anti-inflammatory potential of Montmorency tart cherry juice in adults with mild-to-moderate ulcerative colitis. The study represents one of the few rigorously designed clinical trials to examine a whole-food intervention using objective inflammatory biomarkers in this patient population.
In the trial, participants with clinically stable mild-to-moderate UC were instructed to consume 130 ml of diluted Montmorency tart cherry juice twice daily for a period of six weeks, while continuing their usual medications without any changes. This design allowed the researchers to isolate the effects of the dietary intervention as an adjunct rather than a replacement for conventional treatment. The primary outcome measure was faecal calprotectin, a well-established and widely accepted biomarker of intestinal inflammation that closely reflects mucosal immune activity and disease severity in inflammatory bowel disease.
The results were notable. Participants who consumed the tart cherry juice experienced an average 40% reduction in faecal calprotectin levels compared with baseline, indicating a substantial decrease in intestinal inflammation. Given that faecal calprotectin is considered the gold-standard noninvasive marker for monitoring mucosal inflammation in UC, this reduction is clinically meaningful and suggests a true biological effect rather than a subjective improvement alone. Importantly, this improvement occurred without alterations to prescribed medications, highlighting the potential of tart cherry juice as a supportive dietary strategy.
In addition to biomarker improvements, the study reported a 9% enhancement in disease-related quality of life. Although modest in magnitude, this improvement is significant in a chronic condition where even small gains in daily functioning, symptom burden, and well-being can have meaningful impacts on patients’ lives. The parallel improvement in both objective inflammation and patient-reported outcomes strengthens the overall credibility of the findings.
The researchers attributed these benefits primarily to the high anthocyanin content of Montmorency cherries. Anthocyanins are polyphenolic compounds with well-documented antioxidant and anti-inflammatory properties. Mechanistically, anthocyanins have been shown in prior research to reduce pro-inflammatory cytokine production, inhibit oxidative stress, and modulate gut microbiota composition—all of which are relevant to the pathophysiology of ulcerative colitis. By acting directly at the mucosal level, these compounds may help dampen chronic immune activation in the colon.
While the findings are promising, the authors emphasized that tart cherry juice should not be viewed as a standalone treatment. Instead, its value lies in its role as an adjunctive intervention that may enhance disease control when combined with standard therapy. Larger and longer-term trials will be necessary to confirm durability of the effect, determine optimal dosing, and assess whether benefits extend to patients with more severe disease.
In conclusion, the 2025 randomized, placebo-controlled trial published by researchers from the University of Hertfordshire and the University of Central Lancashire provides strong evidence that Montmorency tart cherry juice can significantly reduce intestinal inflammation and improve quality of life in adults with mild-to-moderate ulcerative colitis (npj Science of Food, 2025). These findings support the growing recognition that targeted dietary strategies rich in bioactive compounds may play an important complementary role in the long-term management of inflammatory bowel disease.
News in the same category

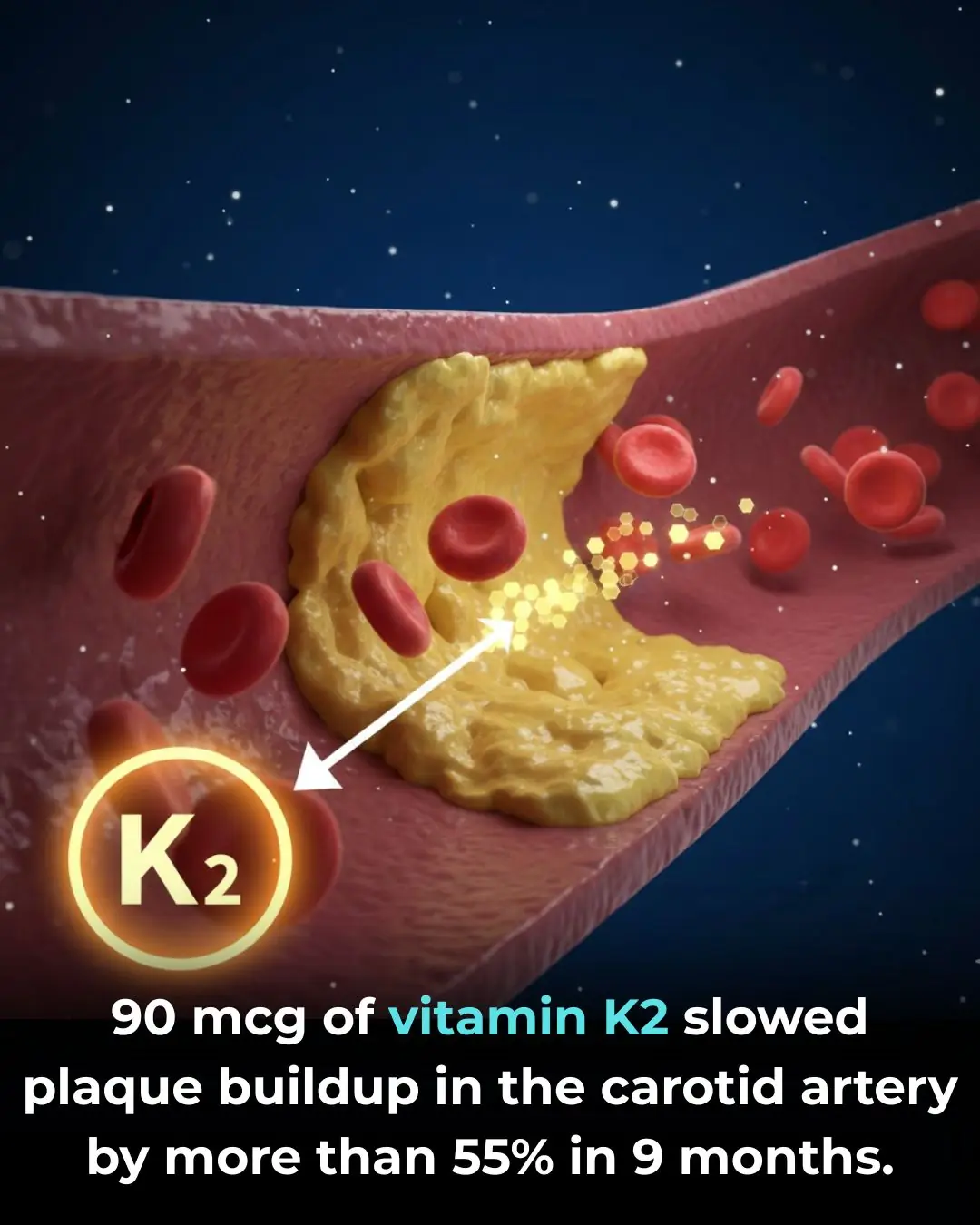
Vitamin K2 Supplementation and Vascular Health in Chronic Kidney Disease

Daily Prune Consumption and Bone Health in Postmenopausal Women
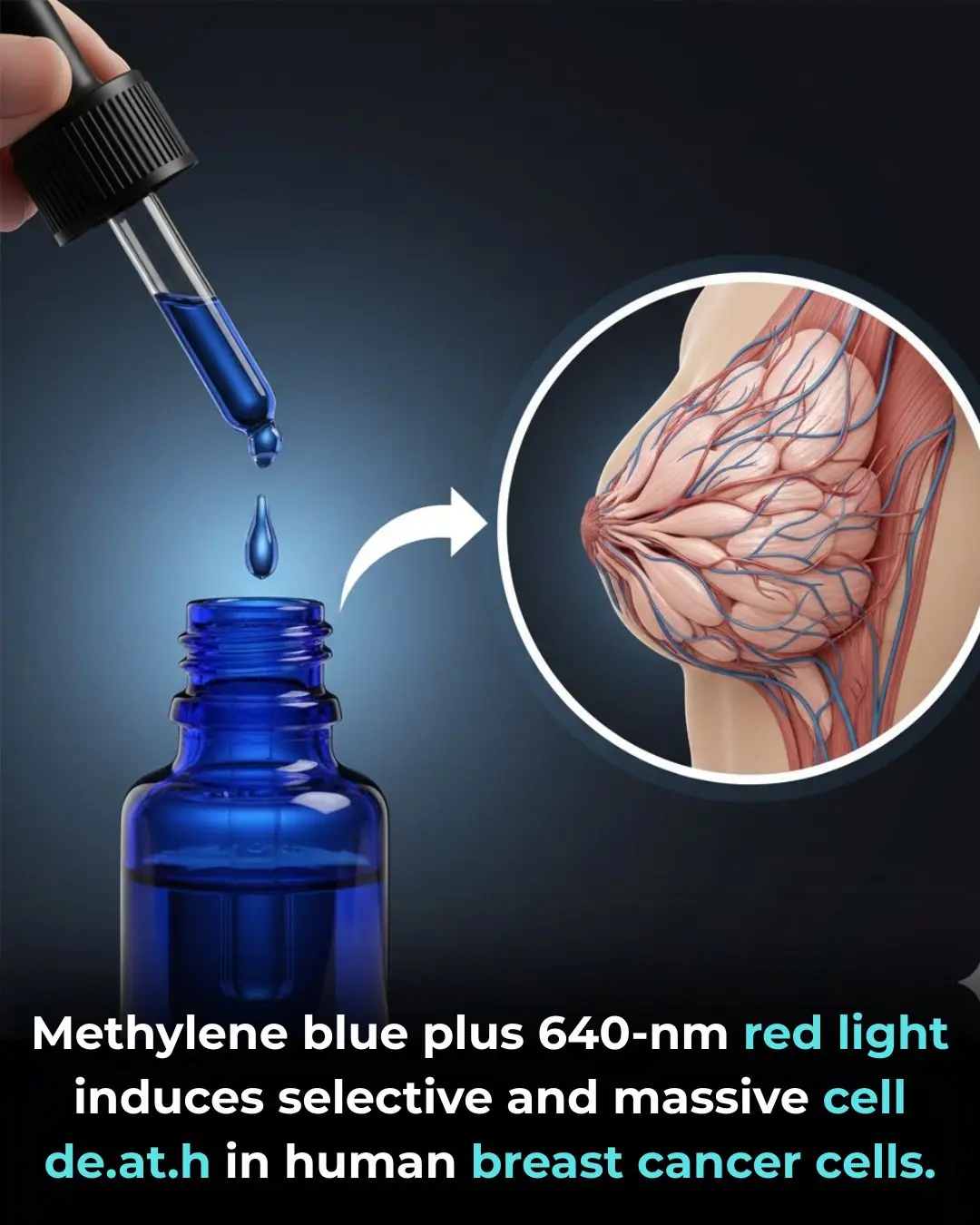
Methylene Blue–Based Photodynamic Therapy as a Selective Strategy Against Breast Cancer Cells
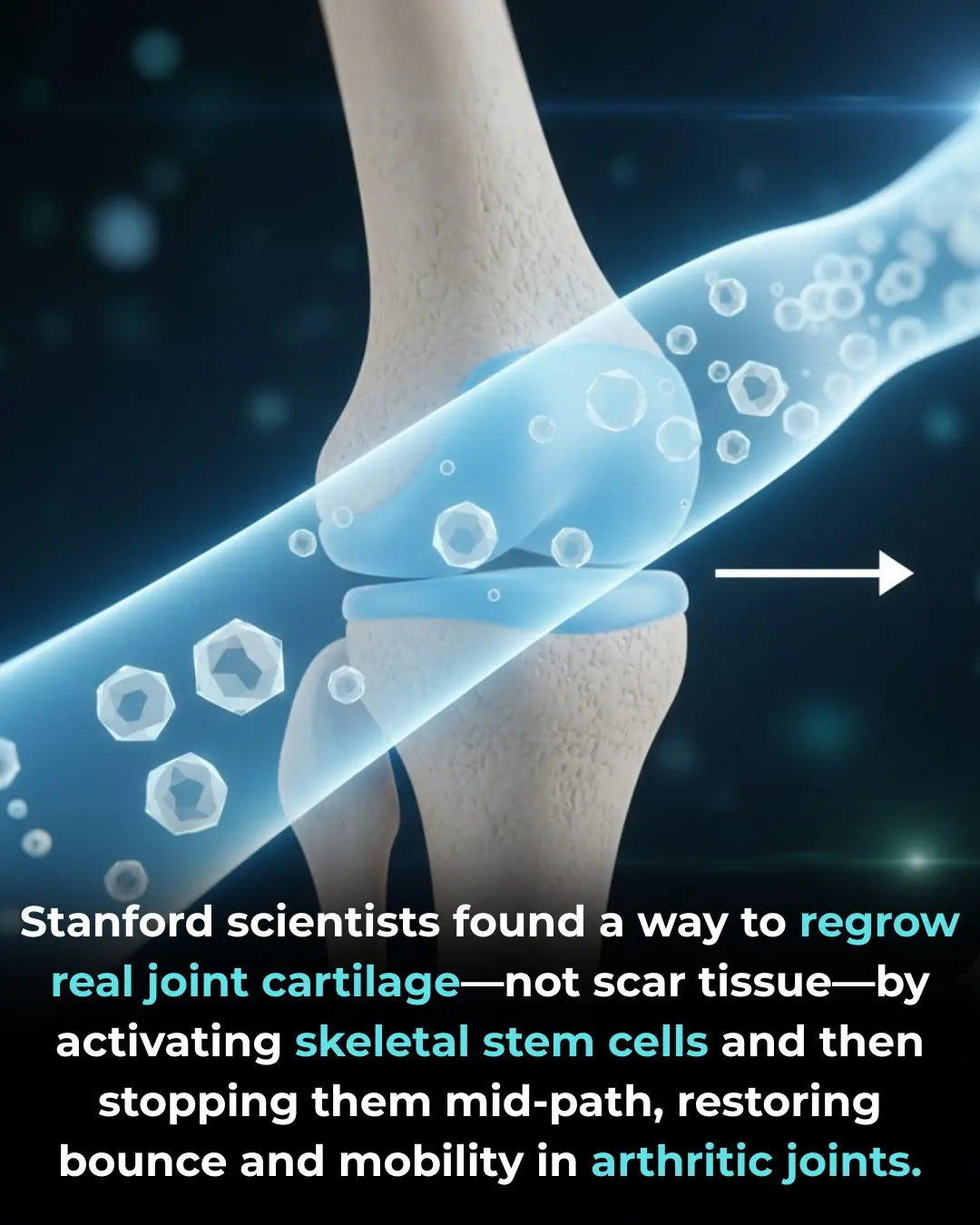
Regenerating True Articular Cartilage: A Breakthrough in Joint Repair

Deuterium-Depleted Water and Long-Term Survival in Cancer Patients: Evidence from a Hungarian Population-Based Analysis

Daily cocoa extract supplements lowered cardiovascular disease deaths by 27%

Ivermectin stopped aggressive cancer cells from moving and spreading in lab tests
The Best Natural Gout Treatments: Remove Uric Acid Crystallization To Prevent Gout And Joint Pain
Quickly Drain You Lymph System Using Theses Simple Techniques to Boost Immunity and Remove Toxins
The Top 20 Essential Oils to Relieve Pain and Inflammation (Research Based)

Headache Above or Behind the Left Eye: Causes and Treatments
Root Canals May Lower Risk of Heart Disease, Diabetes

Mother-to-Infant Microbiome Transmission: Beyond Bacteria to Genes
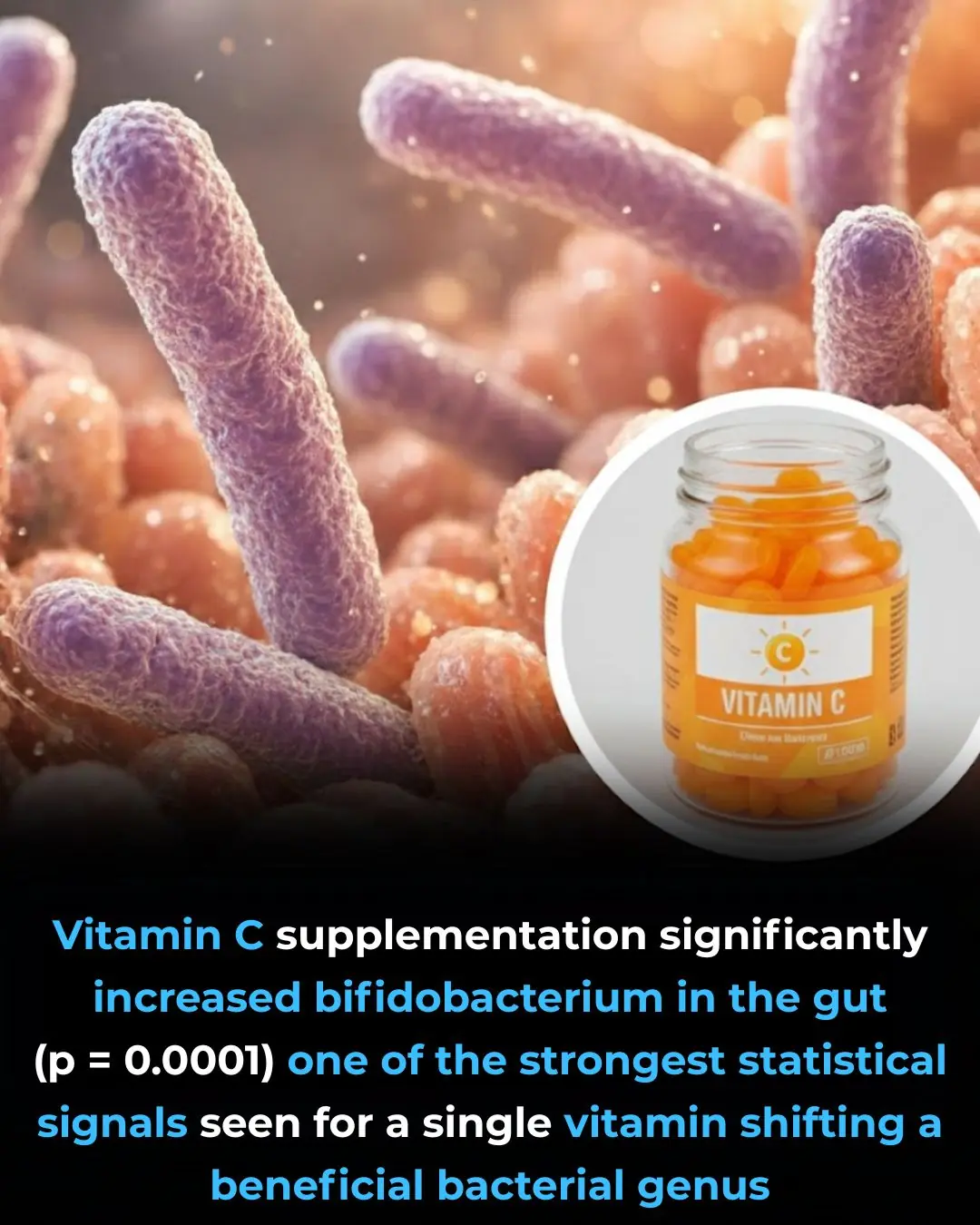
Vitamin C Supplementation and Its Targeted Impact on the Human Gut Microbiome

Sleep and Dementia Risk: What You Should Know

Warning: 4 things to avoid when napping to prevent illness

The Amazing Benefits of Guava Leaf Water That Few People Know

Are Vaccines Doing More Than Just Preventing Infection?
News Post

Australian HPV Vaccination Marks Cervical Cancer Milestone

My outdoor faucet suddenly froze and now I’m seeing water seeping indoors — what should I do before a plumber can come?

Vitamin K2 Supplementation and Vascular Health in Chronic Kidney Disease

Daily Prune Consumption and Bone Health in Postmenopausal Women

Methylene Blue–Based Photodynamic Therapy as a Selective Strategy Against Breast Cancer Cells

Regenerating True Articular Cartilage: A Breakthrough in Joint Repair

Deuterium-Depleted Water and Long-Term Survival in Cancer Patients: Evidence from a Hungarian Population-Based Analysis

Daily cocoa extract supplements lowered cardiovascular disease deaths by 27%

Ivermectin stopped aggressive cancer cells from moving and spreading in lab tests
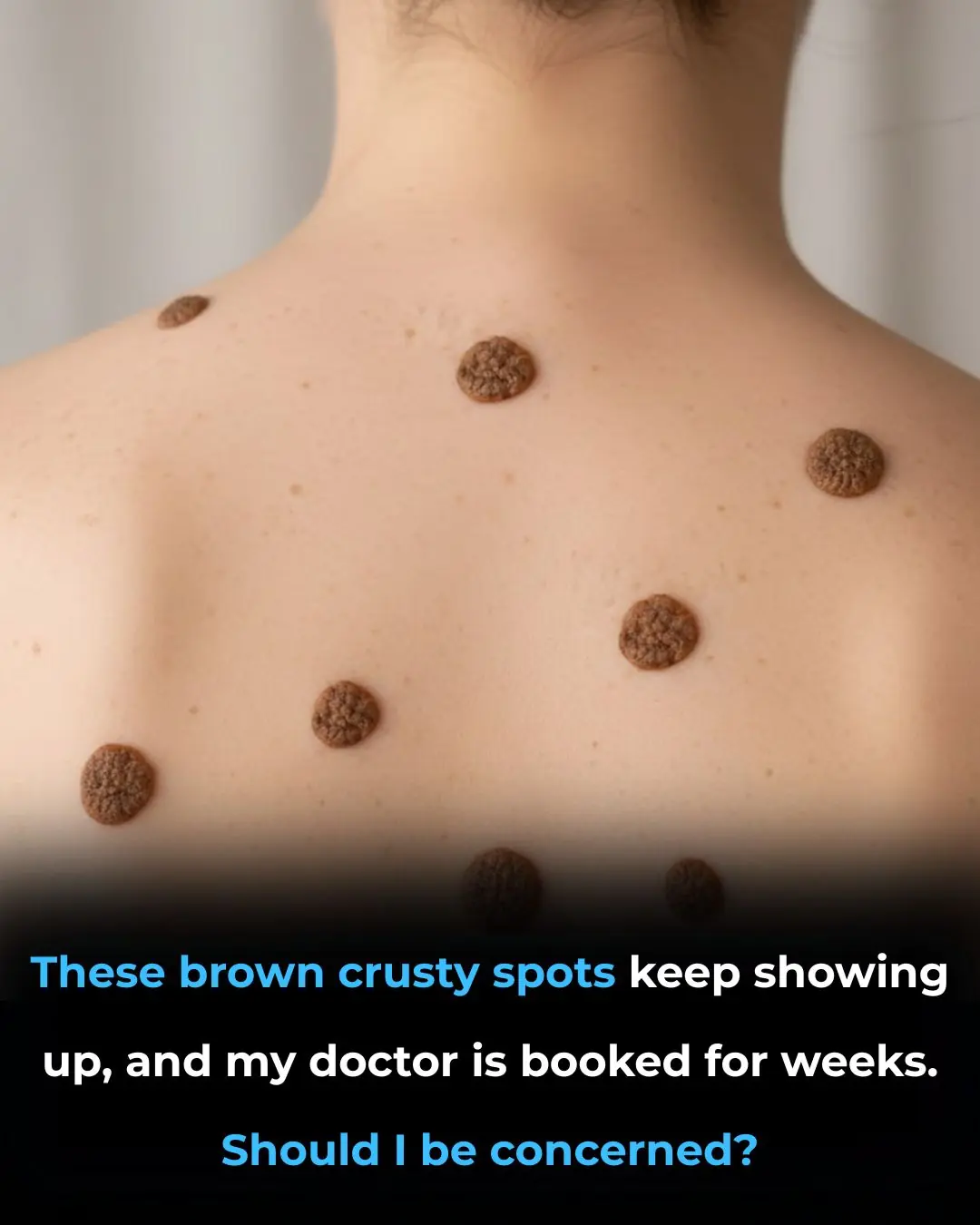
These brown crusty spots keep showing up, and my doctor is booked for weeks given Christmas. Should I be concerned?
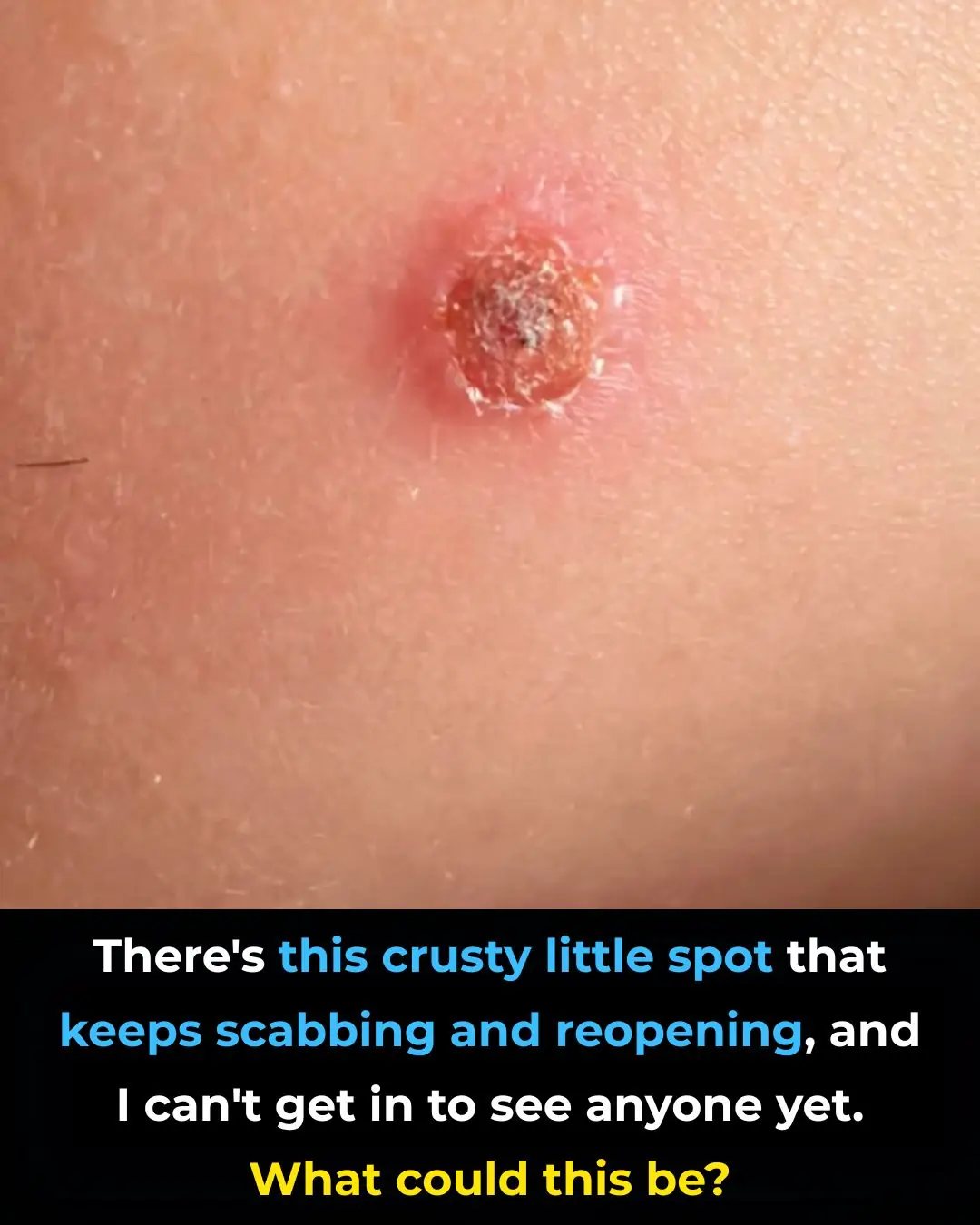
There’s this crusty little spot that keeps scabbing and reopening, and I can’t get in to see anyone yet. What could this be?

I had no clue about this
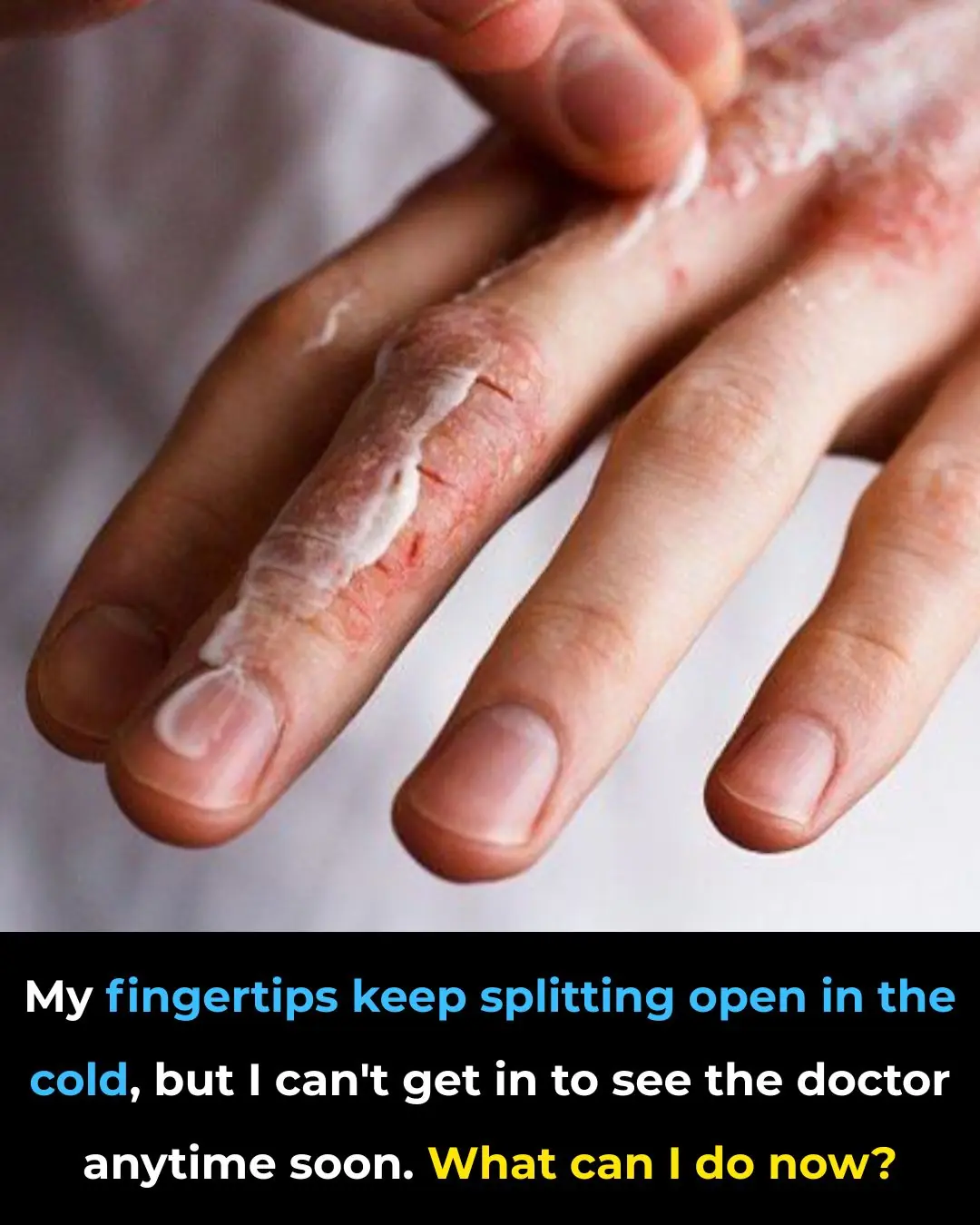
My fingertips keep splitting open in the cold, but I can’t get in to see the doctor anytime soon. What can I do now?

The Best Natural Gout Treatments: Remove Uric Acid Crystallization To Prevent Gout And Joint Pain

Quickly Drain You Lymph System Using Theses Simple Techniques to Boost Immunity and Remove Toxins

The Top 20 Essential Oils to Relieve Pain and Inflammation (Research Based)

Headache Above or Behind the Left Eye: Causes and Treatments

Root Canals May Lower Risk of Heart Disease, Diabetes
