
A Breakthrough in Glioblastoma Immunotherapy: Rapid Tumor Regression with CARv3-TEAM-E
A Breakthrough in Glioblastoma Immunotherapy: Rapid Tumor Regression with CARv3-TEAM-E
Glioblastoma is widely regarded as the most aggressive and lethal primary brain tumor in adults. Despite decades of research and the use of maximal surgery, radiation, and chemotherapy, median survival remains little more than a year. One of the central reasons for this grim prognosis is glioblastoma’s extraordinary ability to evade the immune system. Tumor cells display heterogeneous surface markers, suppress immune activity within the brain, and rapidly adapt to single-target therapies. Against this backdrop, a report from early 2025 describing a near-complete tumor regression within days of treatment represents a potentially transformative moment in neuro-oncology.
Researchers at Massachusetts General Hospital, working in collaboration with Massachusetts Institute of Technology, reported one of the most dramatic responses ever observed in a patient with glioblastoma. A single dose of an experimental chimeric antigen receptor (CAR) T-cell therapy—known as CARv3-TEAM-E—caused a patient’s brain tumor to nearly disappear within just five days. In another treated individual, tumor volume was reduced by approximately 60%, with the response persisting for several months. Such rapid and profound tumor regression is virtually unprecedented in glioblastoma treatment.
CAR T-cell therapy works by collecting a patient’s own T cells and genetically engineering them to recognize and attack cancer cells. While CAR T therapies have revolutionized the treatment of certain blood cancers, they have largely failed in solid tumors, including glioblastoma. The reasons include poor T-cell penetration into tumors, immune suppression within the tumor microenvironment, and antigen escape—where cancer cells survive by losing or altering the targeted marker. The CARv3-TEAM-E platform was specifically designed to overcome these limitations.
Unlike earlier CAR T therapies that target a single tumor antigen, CARv3-TEAM-E is a next-generation, multi-targeted approach. The engineered T cells are designed to recognize multiple tumor-associated markers simultaneously, making it far more difficult for cancer cells to evade immune attack. In addition, the therapy incorporates features intended to enhance local immune activation within the tumor environment, counteracting the strong immunosuppressive signals that glioblastoma typically uses to shut down immune responses.
The clinical responses observed in the first treated patients were both rapid and biologically revealing. Imaging showed dramatic tumor shrinkage within days, indicating that the engineered immune cells were not only reaching the tumor but were highly active once there. The speed of the response suggests a direct and potent immune-mediated destruction of tumor cells rather than a slow cytostatic effect. For a disease in which treatments often produce only marginal slowing of progression, such a response represents a profound shift in what may be biologically achievable.
Nevertheless, the researchers emphasized that these results are preliminary and come from a very small number of patients. Glioblastoma is notoriously heterogeneous, and early dramatic responses do not guarantee long-term disease control. Tumors may eventually recur through new resistance mechanisms, and the durability of CARv3-TEAM-E–induced remissions remains unknown. Safety is another critical consideration, as powerful immune activation in the brain carries risks of inflammation, swelling, and neurological complications that must be carefully managed.
Despite these uncertainties, the implications of this work are substantial. This study provides proof of principle that glioblastoma is not inherently immune-proof and that its defenses can be breached with sufficiently sophisticated immunotherapy. By demonstrating that a single infusion of engineered T cells can cause near-complete tumor regression within days, the CARv3-TEAM-E approach challenges long-standing assumptions about the limits of brain cancer treatment.
In conclusion, the early 2025 report from Massachusetts General Hospital and MIT describes an extraordinary clinical response to a next-generation CAR T-cell therapy in glioblastoma (Massachusetts General Hospital / MIT collaborative report, 2025). While far from a cure and still in the earliest stages of clinical testing, this work represents a landmark advance in cancer immunotherapy. If future trials confirm safety and durability, multi-targeted CAR T-cell strategies like CARv3-TEAM-E could redefine the therapeutic landscape for one of the deadliest human cancers.
News in the same category


Pomegranate Seed Oil Supplementation and Cognitive Improvement in Mild Cognitive Impairment

Selective Anti-Cancer Effects of Frankincense: Evidence from Laboratory Studies

Kimchi Consumption and Immune Balance: Evidence from a 12-Week Human Clinical Study

Selective Anti-Cancer Activity of Dandelion Root Extract in Colorectal Cancer
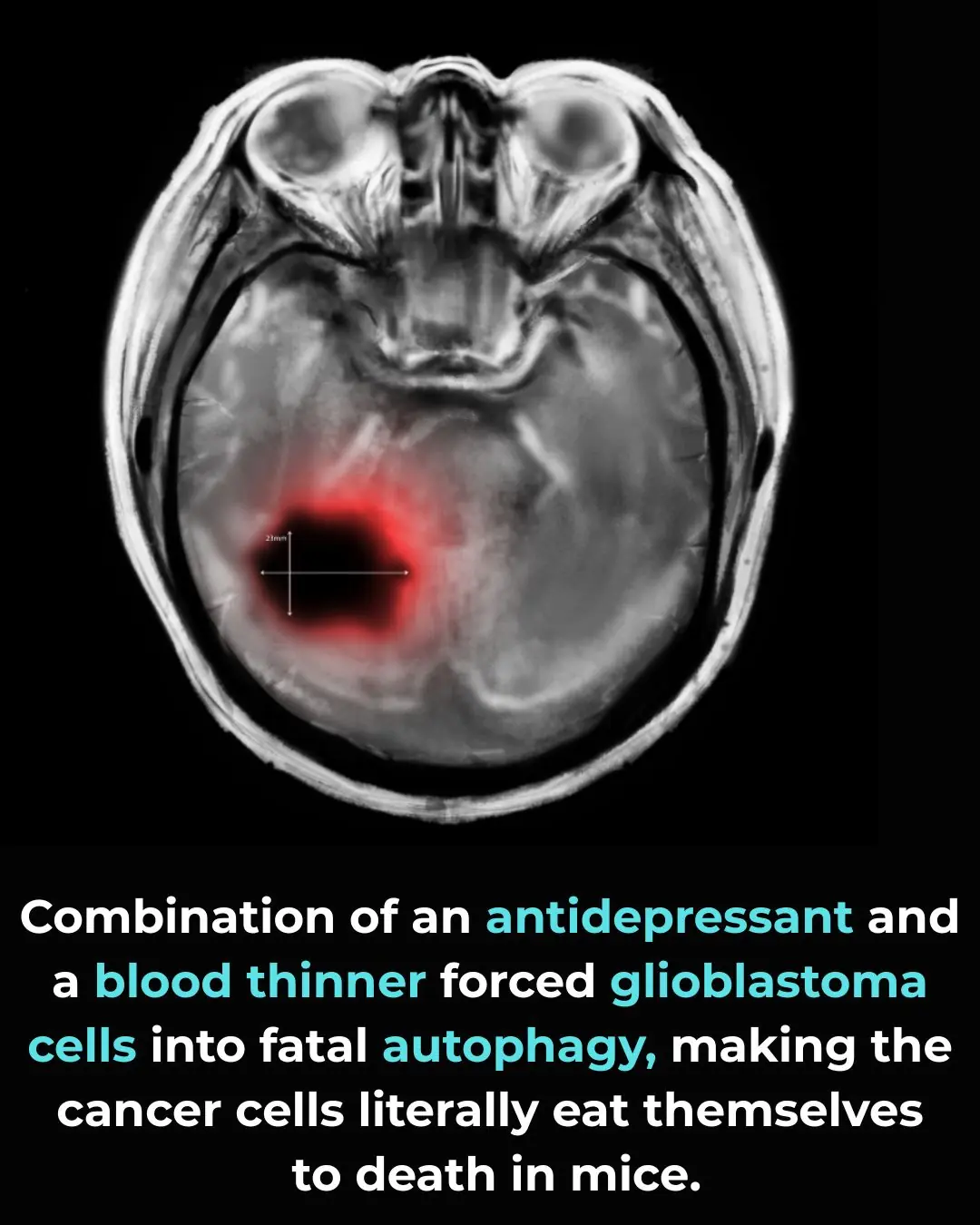
Inducing Lethal Autophagy in Glioblastoma Through Drug Repurposing
mRNA Flu Vaccines Show Higher Effectiveness Than Traditional Quadrivalent Shots, Phase 3 Trial Finds
Early Signs of Multiple Sclerosis

11 Benefits of Going Caffeine-Free
5 Early Signs of Cervical Cancer That Are Often Ignored: 90% of Women Overlook Them

The Cheap Drink That Can Help Prevent Stroke, Reduce Blood Fat, and Fight Cancer
8 Signs You’re Eating Too Much Sugar

Accidental discovery of bone-eroding cancer after a fall: It turns out the body had been crying for help for a long time but was ignored
10 Eye Symptoms to Watch Out For

Does Eating Bananas Before Bed Have Any Benefits?

Can’t Fall Back Asleep After Waking Up to Use the Bathroom? Try These 5 Hacks

What Is Preventive Botox (or ‘Baby Botox’) — and Is It Safe?

Do You Need a Vitamin D Supplement? Everything to Know

Why Does My Heart Hurt? Common Reasons For Heart or Chest Pain
News Post

Don’t Soak Frozen Meat in Cold Water: A Chef’s 5-Minute Thawing Trick That Keeps Meat Tasty and Nutritious

Pomegranate Seed Oil Supplementation and Cognitive Improvement in Mild Cognitive Impairment

Selective Anti-Cancer Effects of Frankincense: Evidence from Laboratory Studies

Kimchi Consumption and Immune Balance: Evidence from a 12-Week Human Clinical Study

Selective Anti-Cancer Activity of Dandelion Root Extract in Colorectal Cancer

Inducing Lethal Autophagy in Glioblastoma Through Drug Repurposing

WHO Recommends GLP-1 Therapies for Obesity Management in Landmark New Guidance

mRNA Flu Vaccines Show Higher Effectiveness Than Traditional Quadrivalent Shots, Phase 3 Trial Finds

A cold draft keeps sneaking in under my front door, and the handyman can’t come until after the holidays. What can I do right now?

The 30-Minute Rule Everyone Needs to Know
These red patches flare up every night, but my doc can’t see me until next month. Any idea what’s happening?

Early Signs of Multiple Sclerosis

11 Benefits of Going Caffeine-Free

5 Early Signs of Cervical Cancer That Are Often Ignored: 90% of Women Overlook Them

The Cheap Drink That Can Help Prevent Stroke, Reduce Blood Fat, and Fight Cancer

Bone-chilling 2025 predictions from both Nostradamus and Baba Vanga

2 quick and easy ways to wash yellowed pillow inserts, making them sparkling white like new in no time

Yellowed, burnt-on stainless steel pots will shine like new after soaking them in this water
