
Inducing Lethal Autophagy in Glioblastoma Through Drug Repurposing
Inducing Lethal Autophagy in Glioblastoma Through Drug Repurposing
Glioblastoma is the most aggressive and lethal form of primary brain cancer, characterized by rapid growth, extensive infiltration into surrounding brain tissue, and near-universal recurrence despite surgery, radiation, and chemotherapy. Median survival remains approximately 15 months, underscoring the urgent need for new therapeutic strategies that attack glioblastoma through fundamentally different biological mechanisms. In this context, a study published in Cancer Cell by Douglas Hanahan and colleagues at the Swiss Federal Institute of Technology Lausanne (EPFL) revealed a striking vulnerability in glioblastoma cells: their susceptibility to being driven into self-destructive autophagy.
Autophagy is a tightly regulated cellular recycling process that allows cells to survive stress by breaking down damaged organelles and proteins. In cancer, autophagy often plays a paradoxical role. At moderate levels, it helps tumor cells survive nutrient deprivation, hypoxia, and therapy-induced stress. However, when autophagy is excessively activated, it can cross a threshold and become lethal, causing cells to digest essential components and die. The EPFL-led team hypothesized that glioblastoma cells might be uniquely sensitive to this form of “hyper-autophagy” if multiple regulatory checkpoints were disrupted simultaneously.
To test this idea, the researchers implanted mice with early-stage human glioblastoma tumors and treated them with two repurposed drugs: a tricyclic antidepressant and an anticoagulant (blood-thinner). Individually, each drug had little or no effect on tumor growth or survival. Remarkably, when administered together, the drugs acted synergistically to overwhelm the tumor cells’ autophagic control systems. Instead of promoting survival, autophagy became excessive and destructive, causing the cancer cells to digest themselves from within.
At the cellular level, the combination therapy disrupted distinct but complementary components of the autophagic regulatory circuitry. By simultaneously removing both “brakes” and “balancers” of the autophagy system, the treatment forced glioblastoma cells into an irreversible state of hyper-autophagy. This effect was highly selective for tumor cells, which are already under extreme metabolic and proteotoxic stress, making them far more vulnerable than normal brain cells to runaway self-digestion.
The therapeutic impact in animal models was substantial. Tumor growth was significantly slowed, and median survival time in treated mice was approximately doubled compared with controls. Although the treatment did not eradicate tumors or cure the disease, the magnitude of the survival benefit was notable given the aggressiveness of glioblastoma and the limited efficacy of most experimental interventions. Importantly, the study demonstrated that inducing cell-lethal autophagy is a viable anti-cancer strategy rather than merely a theoretical concept.
The broader significance of this work lies in its innovative use of drug repurposing. Both compounds used in the study are already approved for other indications, meaning their pharmacology and safety profiles are relatively well characterized. This raises the possibility—though not the guarantee—that translation to clinical testing could be faster than for entirely new drugs. Moreover, the study shifts the therapeutic paradigm away from directly blocking tumor growth signals and toward exploiting intrinsic stress-response systems that cancer cells depend on for survival.
Nevertheless, the authors emphasized that the findings remain preclinical. The experiments were conducted exclusively in mouse models, and glioblastoma biology in humans is notoriously complex. Optimal dosing, safety of the drug combination in patients, interactions with standard treatments, and the risk of unintended effects on normal brain cells all remain unresolved. As such, the approach cannot yet be considered a viable treatment for patients.
In conclusion, the study “Dual targeting of the autophagic regulatory circuitry in gliomas with repurposed drugs elicits cell-lethal autophagy and therapeutic benefit” published in Cancer Cell demonstrates that glioblastoma cells can be pushed into fatal self-digestion by simultaneously disrupting key autophagy regulators (Cancer Cell, year of publication). While still untested in humans, this work uncovers a powerful new therapeutic concept and highlights autophagy as a promising target in one of the most treatment-resistant cancers known.
News in the same category

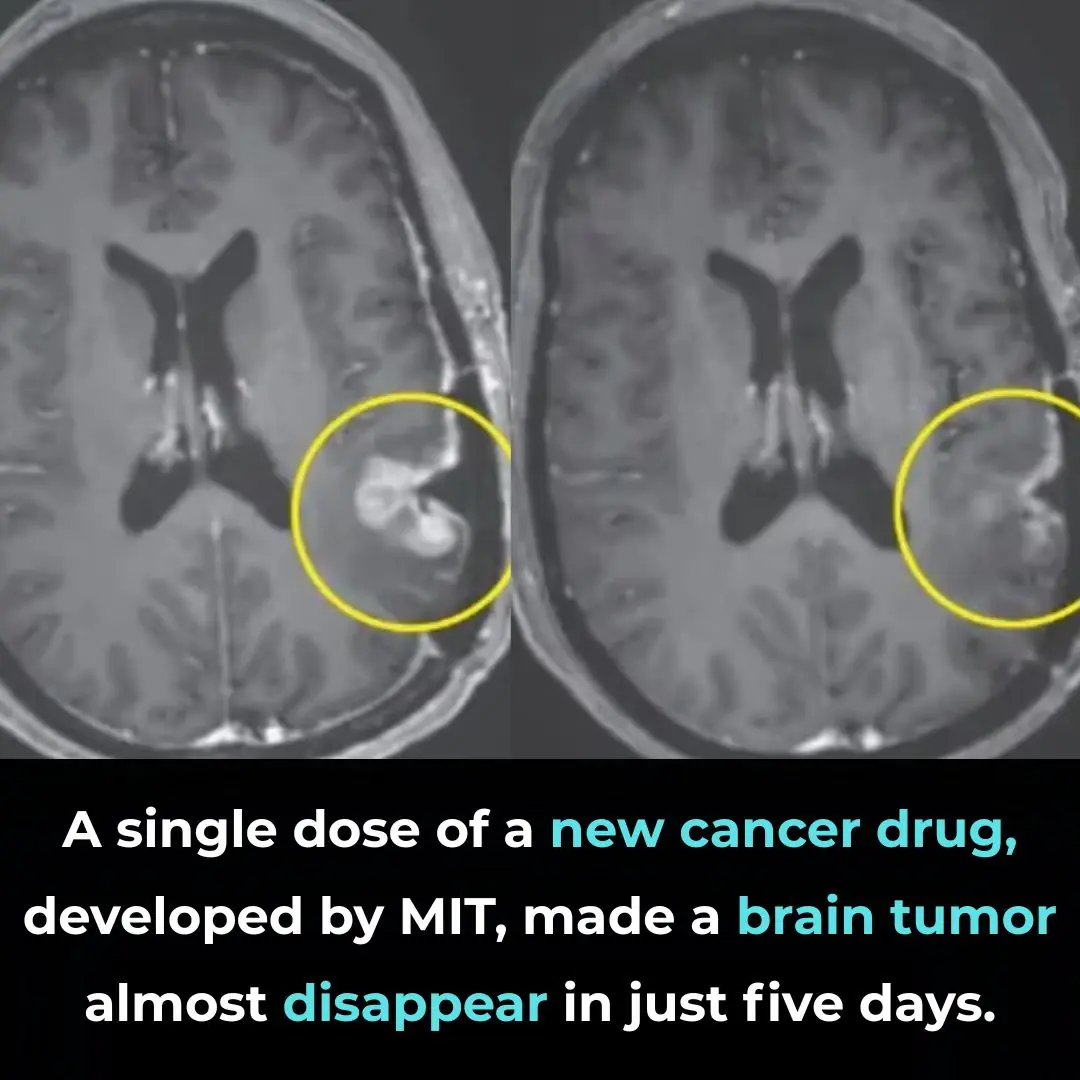
A Breakthrough in Glioblastoma Immunotherapy: Rapid Tumor Regression with CARv3-TEAM-E

Pomegranate Seed Oil Supplementation and Cognitive Improvement in Mild Cognitive Impairment

Selective Anti-Cancer Effects of Frankincense: Evidence from Laboratory Studies

Kimchi Consumption and Immune Balance: Evidence from a 12-Week Human Clinical Study

Selective Anti-Cancer Activity of Dandelion Root Extract in Colorectal Cancer
mRNA Flu Vaccines Show Higher Effectiveness Than Traditional Quadrivalent Shots, Phase 3 Trial Finds
Early Signs of Multiple Sclerosis

11 Benefits of Going Caffeine-Free
5 Early Signs of Cervical Cancer That Are Often Ignored: 90% of Women Overlook Them

The Cheap Drink That Can Help Prevent Stroke, Reduce Blood Fat, and Fight Cancer
8 Signs You’re Eating Too Much Sugar

Accidental discovery of bone-eroding cancer after a fall: It turns out the body had been crying for help for a long time but was ignored
10 Eye Symptoms to Watch Out For

Does Eating Bananas Before Bed Have Any Benefits?

Can’t Fall Back Asleep After Waking Up to Use the Bathroom? Try These 5 Hacks

What Is Preventive Botox (or ‘Baby Botox’) — and Is It Safe?

Do You Need a Vitamin D Supplement? Everything to Know

Why Does My Heart Hurt? Common Reasons For Heart or Chest Pain
News Post

Don’t Soak Frozen Meat in Cold Water: A Chef’s 5-Minute Thawing Trick That Keeps Meat Tasty and Nutritious

A Breakthrough in Glioblastoma Immunotherapy: Rapid Tumor Regression with CARv3-TEAM-E

Pomegranate Seed Oil Supplementation and Cognitive Improvement in Mild Cognitive Impairment

Selective Anti-Cancer Effects of Frankincense: Evidence from Laboratory Studies

Kimchi Consumption and Immune Balance: Evidence from a 12-Week Human Clinical Study

Selective Anti-Cancer Activity of Dandelion Root Extract in Colorectal Cancer

WHO Recommends GLP-1 Therapies for Obesity Management in Landmark New Guidance

mRNA Flu Vaccines Show Higher Effectiveness Than Traditional Quadrivalent Shots, Phase 3 Trial Finds

A cold draft keeps sneaking in under my front door, and the handyman can’t come until after the holidays. What can I do right now?

The 30-Minute Rule Everyone Needs to Know
These red patches flare up every night, but my doc can’t see me until next month. Any idea what’s happening?

Early Signs of Multiple Sclerosis

11 Benefits of Going Caffeine-Free

5 Early Signs of Cervical Cancer That Are Often Ignored: 90% of Women Overlook Them

The Cheap Drink That Can Help Prevent Stroke, Reduce Blood Fat, and Fight Cancer

Bone-chilling 2025 predictions from both Nostradamus and Baba Vanga

2 quick and easy ways to wash yellowed pillow inserts, making them sparkling white like new in no time

Yellowed, burnt-on stainless steel pots will shine like new after soaking them in this water
