mRNA Flu Vaccines Show Higher Effectiveness Than Traditional Quadrivalent Shots, Phase 3 Trial Finds
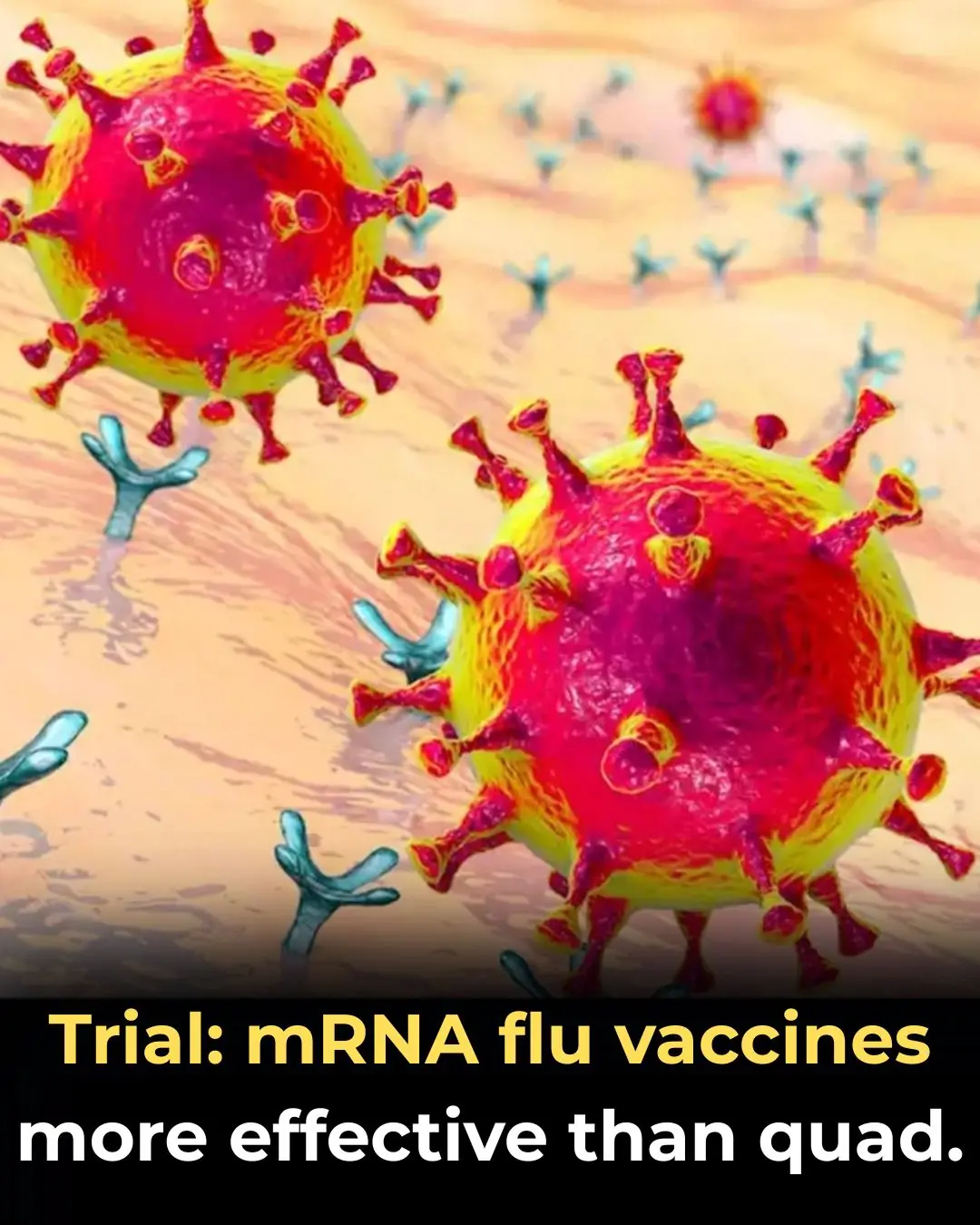
A new phase 3 randomized clinical trial suggests that mRNA-based influenza vaccines may offer significantly stronger protection than conventional inactivated quadrivalent flu vaccines. According to the data, the mRNA vaccine demonstrated approximately 35% greater effectiveness against two circulating influenza strains, marking an important step forward in seasonal flu prevention.
The findings add to growing interest in mRNA technology beyond COVID-19 and highlight its potential to transform how influenza vaccines are developed, updated, and deployed each year.
Understanding the Study at a Glance
The trial was a large, randomized, phase 3 study, designed to directly compare an investigational mRNA influenza vaccine with a standard inactivated quadrivalent influenza vaccine (QIV). Participants were randomly assigned to receive either vaccine and were followed through the flu season to assess laboratory-confirmed influenza infections.
Key findings included:
-
The mRNA vaccine reduced the risk of influenza infection by about 35% more than the quadrivalent inactivated vaccine.
-
Improved protection was observed across two distinct influenza strains, suggesting broader immune coverage.
-
The safety profile of the mRNA vaccine was comparable to existing flu vaccines, with mostly mild to moderate side effects.
Why Quadrivalent Flu Vaccines Have Limitations
Traditional quadrivalent flu vaccines are designed to protect against four influenza strains—typically two influenza A strains and two influenza B strains. While these vaccines have saved countless lives, they face ongoing challenges:
-
Egg-based production delays, which can reduce accuracy if circulating strains shift.
-
Mismatch risk between vaccine strains and real-world viruses.
-
Variable effectiveness, often ranging from 40% to 60% in a good year.
These limitations have driven scientists to explore faster, more adaptable vaccine platforms.
How mRNA Flu Vaccines Work Differently
mRNA vaccines use a fundamentally different approach. Instead of injecting inactivated virus particles, they deliver messenger RNA instructions that tell the body’s cells to temporarily produce specific influenza proteins. This process triggers a strong immune response without exposure to the live virus.
Advantages of mRNA flu vaccines include:
-
Faster development and updates, allowing rapid adjustment to emerging strains
-
More precise antigen targeting
-
Potentially stronger and more consistent immune responses
-
Scalable manufacturing, independent of eggs or cell cultures
These benefits likely contributed to the improved effectiveness observed in the trial.
What “35% More Effective” Actually Means
A 35% improvement does not mean the vaccine is 35% effective overall. Instead, it means that compared with the standard quadrivalent flu shot, the mRNA vaccine reduced the risk of confirmed flu illness by an additional 35%.
In practical terms, this could translate to:
-
Fewer breakthrough infections
-
Reduced severity of illness
-
Lower rates of flu-related complications, hospitalizations, and missed work or school days
Even modest gains in flu vaccine effectiveness can have a major public health impact when applied across millions of people.
Safety and Tolerability
The trial also carefully evaluated safety outcomes. Reported side effects were similar to those seen with existing flu vaccines and other mRNA vaccines.
Common reactions included:
-
Injection-site pain
-
Fatigue
-
Headache
-
Mild muscle aches
Serious adverse events were rare and occurred at similar rates in both vaccine groups, supporting the overall safety of the mRNA flu vaccine platform.
Implications for Future Flu Seasons
If further data continue to support these results, mRNA flu vaccines could represent a major shift in influenza prevention strategies. Potential long-term benefits include:
-
Better protection for high-risk groups such as older adults and people with chronic illnesses
-
More accurate strain matching year to year
-
Faster response to unexpected viral mutations
-
Reduced global flu burden and healthcare strain
Researchers are also exploring whether mRNA technology could enable multivalent or universal flu vaccines, capable of protecting against a wider range of influenza variants over multiple seasons.
What Comes Next?
Regulatory review, additional real-world studies, and post-marketing surveillance will be essential before mRNA flu vaccines become widely available. Scientists are also investigating how these vaccines perform across different age groups and whether booster strategies may further enhance protection.
A Promising Step Forward
The phase 3 trial results provide compelling evidence that mRNA influenza vaccines may outperform traditional quadrivalent flu shots, offering stronger and more reliable protection against seasonal influenza. While more work lies ahead, this innovation signals a promising future in the fight against one of the world’s most persistent and unpredictable viruses.
As mRNA technology continues to evolve, the annual flu shot—long considered routine—may soon become far more effective than ever before.
News in the same category


Pomegranate Seed Oil Supplementation and Cognitive Improvement in Mild Cognitive Impairment

Selective Anti-Cancer Effects of Frankincense: Evidence from Laboratory Studies

Kimchi Consumption and Immune Balance: Evidence from a 12-Week Human Clinical Study

Selective Anti-Cancer Activity of Dandelion Root Extract in Colorectal Cancer
Inducing Lethal Autophagy in Glioblastoma Through Drug Repurposing
Early Signs of Multiple Sclerosis

11 Benefits of Going Caffeine-Free
5 Early Signs of Cervical Cancer That Are Often Ignored: 90% of Women Overlook Them

The Cheap Drink That Can Help Prevent Stroke, Reduce Blood Fat, and Fight Cancer
8 Signs You’re Eating Too Much Sugar

Accidental discovery of bone-eroding cancer after a fall: It turns out the body had been crying for help for a long time but was ignored
10 Eye Symptoms to Watch Out For

Does Eating Bananas Before Bed Have Any Benefits?

Can’t Fall Back Asleep After Waking Up to Use the Bathroom? Try These 5 Hacks

What Is Preventive Botox (or ‘Baby Botox’) — and Is It Safe?

Do You Need a Vitamin D Supplement? Everything to Know

Why Does My Heart Hurt? Common Reasons For Heart or Chest Pain
Red Spots on Skin: Causes, Treatments and More (Extensive Guide)
News Post

A Breakthrough in Glioblastoma Immunotherapy: Rapid Tumor Regression with CARv3-TEAM-E

Pomegranate Seed Oil Supplementation and Cognitive Improvement in Mild Cognitive Impairment

Selective Anti-Cancer Effects of Frankincense: Evidence from Laboratory Studies

Kimchi Consumption and Immune Balance: Evidence from a 12-Week Human Clinical Study

Selective Anti-Cancer Activity of Dandelion Root Extract in Colorectal Cancer

Inducing Lethal Autophagy in Glioblastoma Through Drug Repurposing

WHO Recommends GLP-1 Therapies for Obesity Management in Landmark New Guidance

A cold draft keeps sneaking in under my front door, and the handyman can’t come until after the holidays. What can I do right now?

The 30-Minute Rule Everyone Needs to Know
These red patches flare up every night, but my doc can’t see me until next month. Any idea what’s happening?

Early Signs of Multiple Sclerosis

11 Benefits of Going Caffeine-Free

5 Early Signs of Cervical Cancer That Are Often Ignored: 90% of Women Overlook Them

The Cheap Drink That Can Help Prevent Stroke, Reduce Blood Fat, and Fight Cancer

Bone-chilling 2025 predictions from both Nostradamus and Baba Vanga

2 quick and easy ways to wash yellowed pillow inserts, making them sparkling white like new in no time

Yellowed, burnt-on stainless steel pots will shine like new after soaking them in this water

8 Signs You’re Eating Too Much Sugar